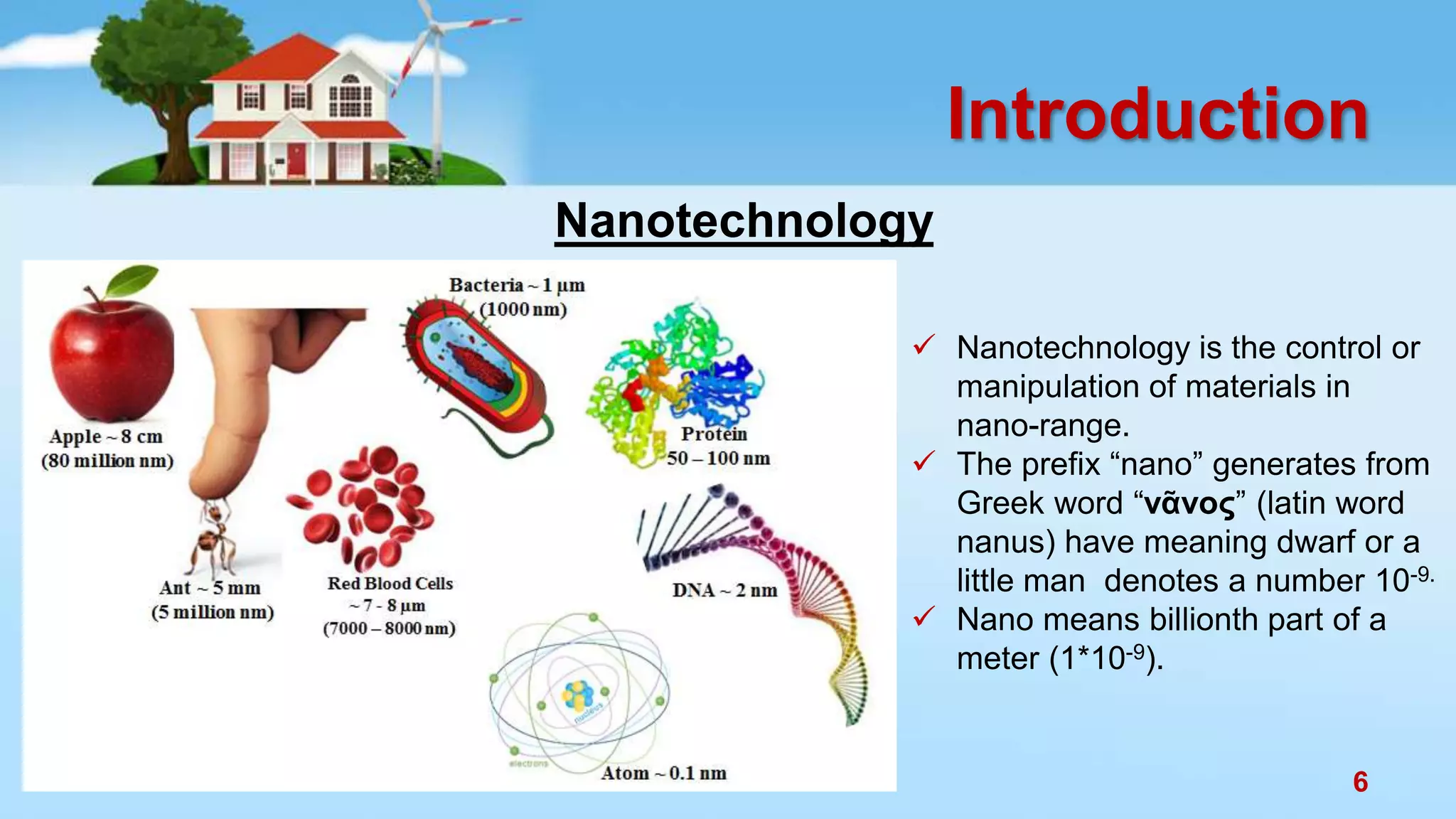
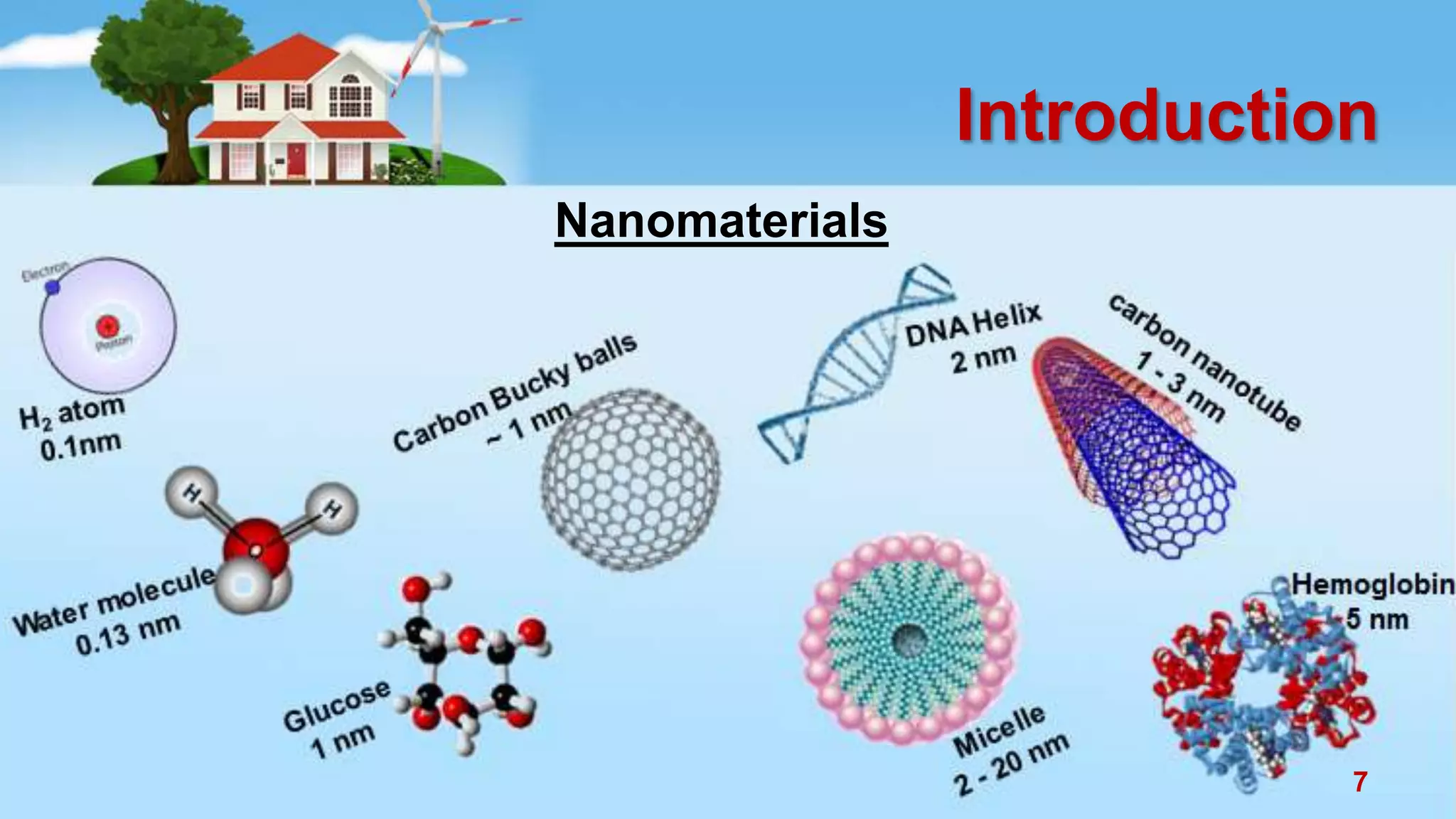
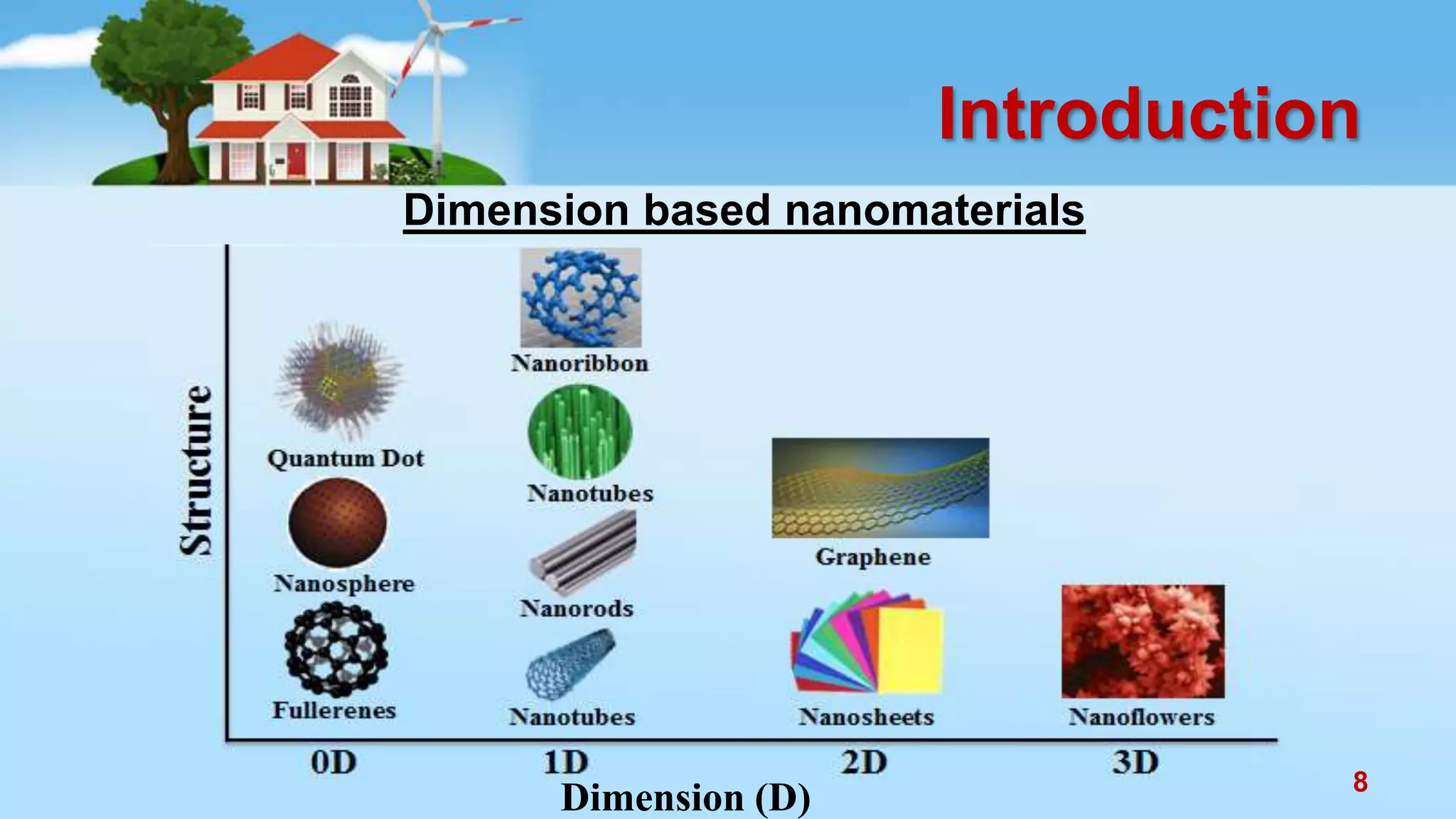
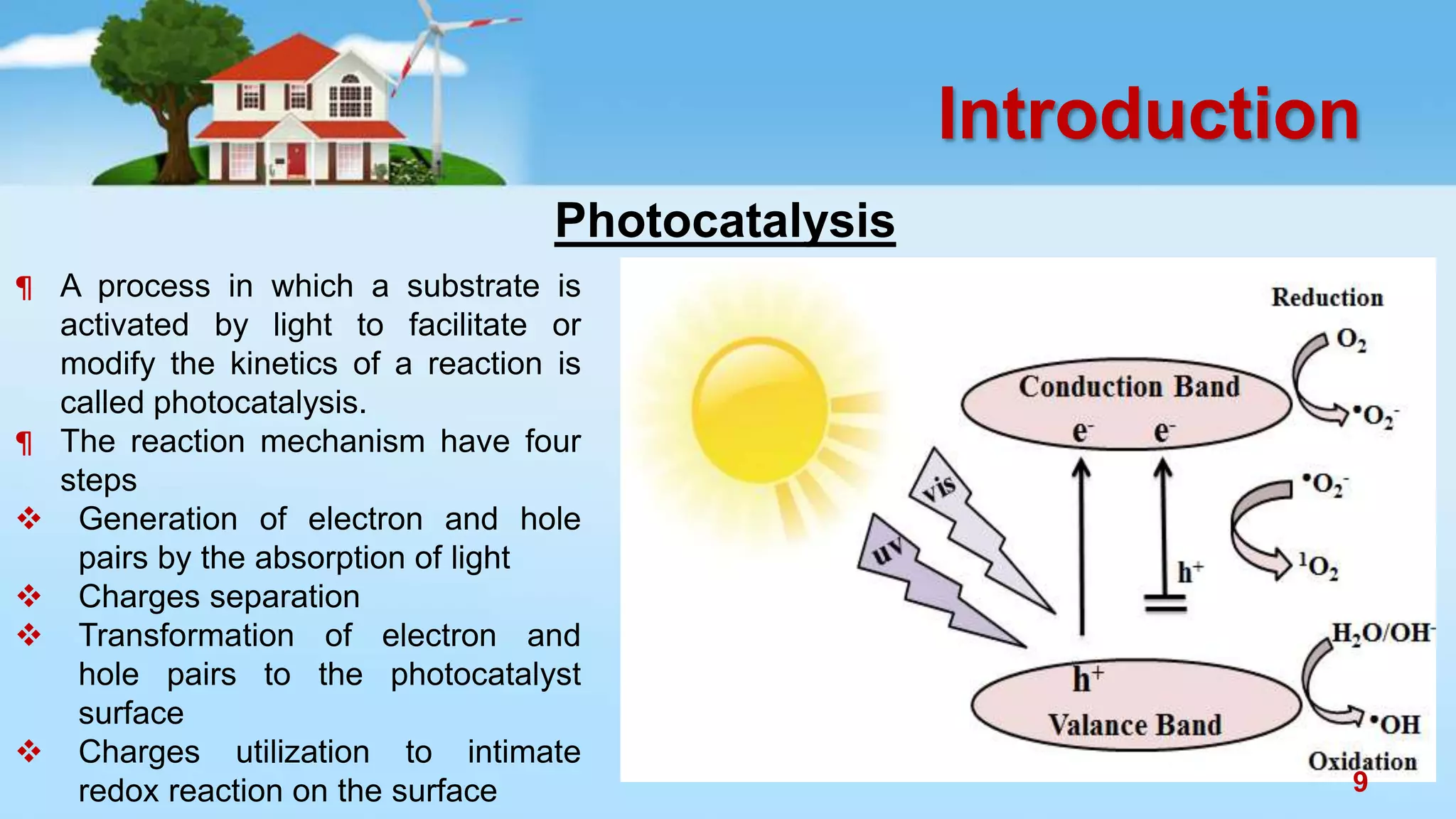
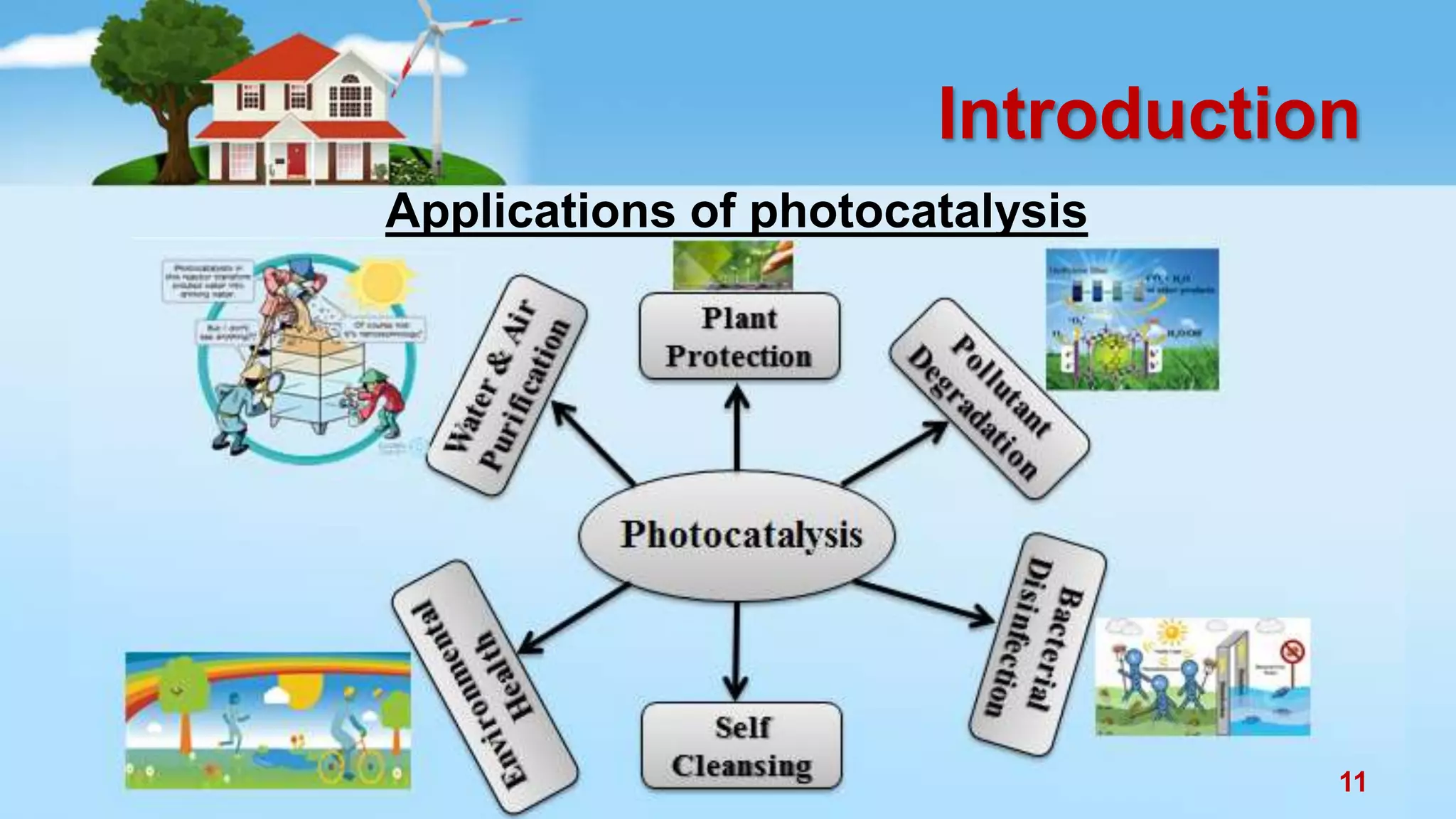
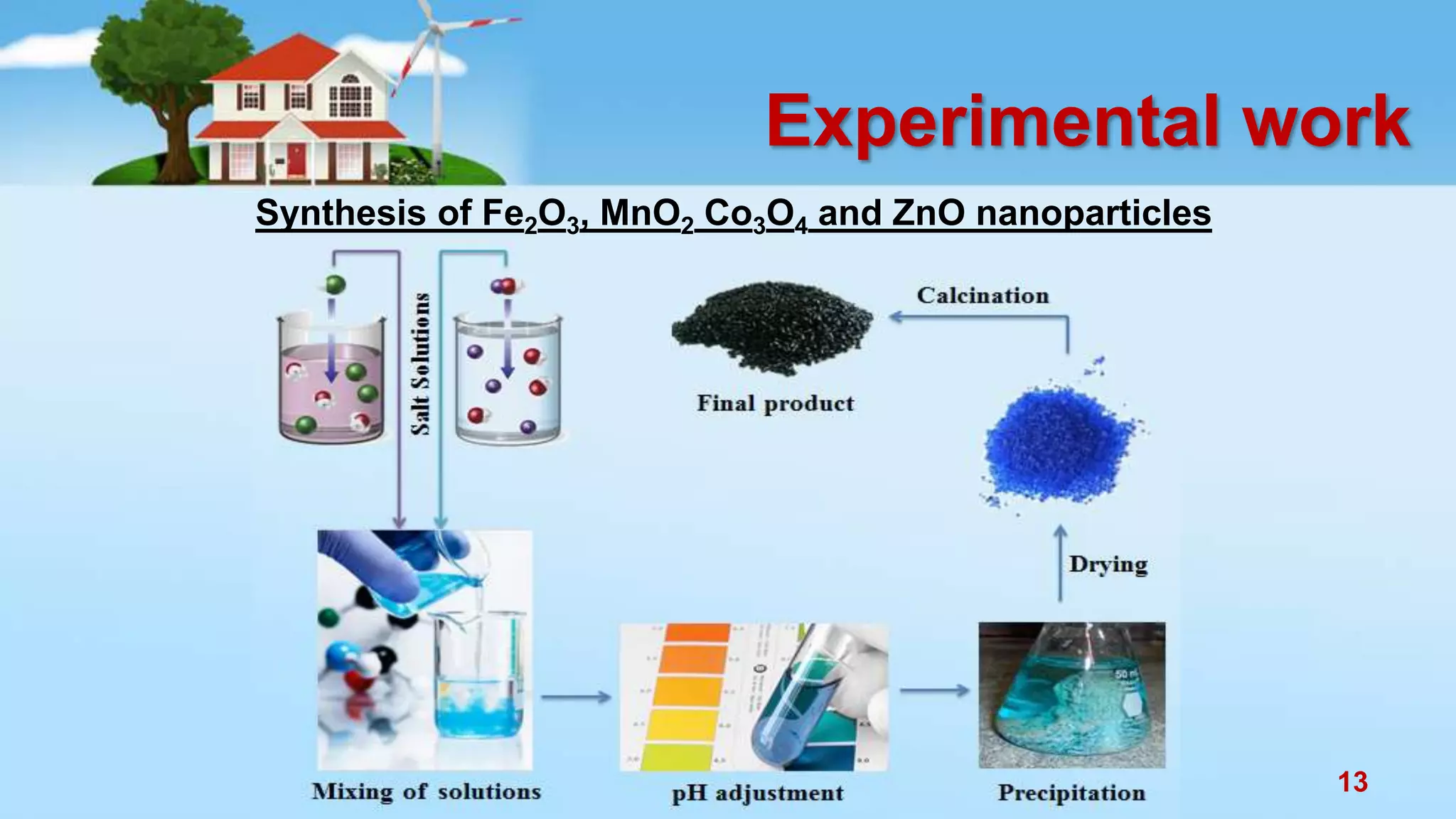
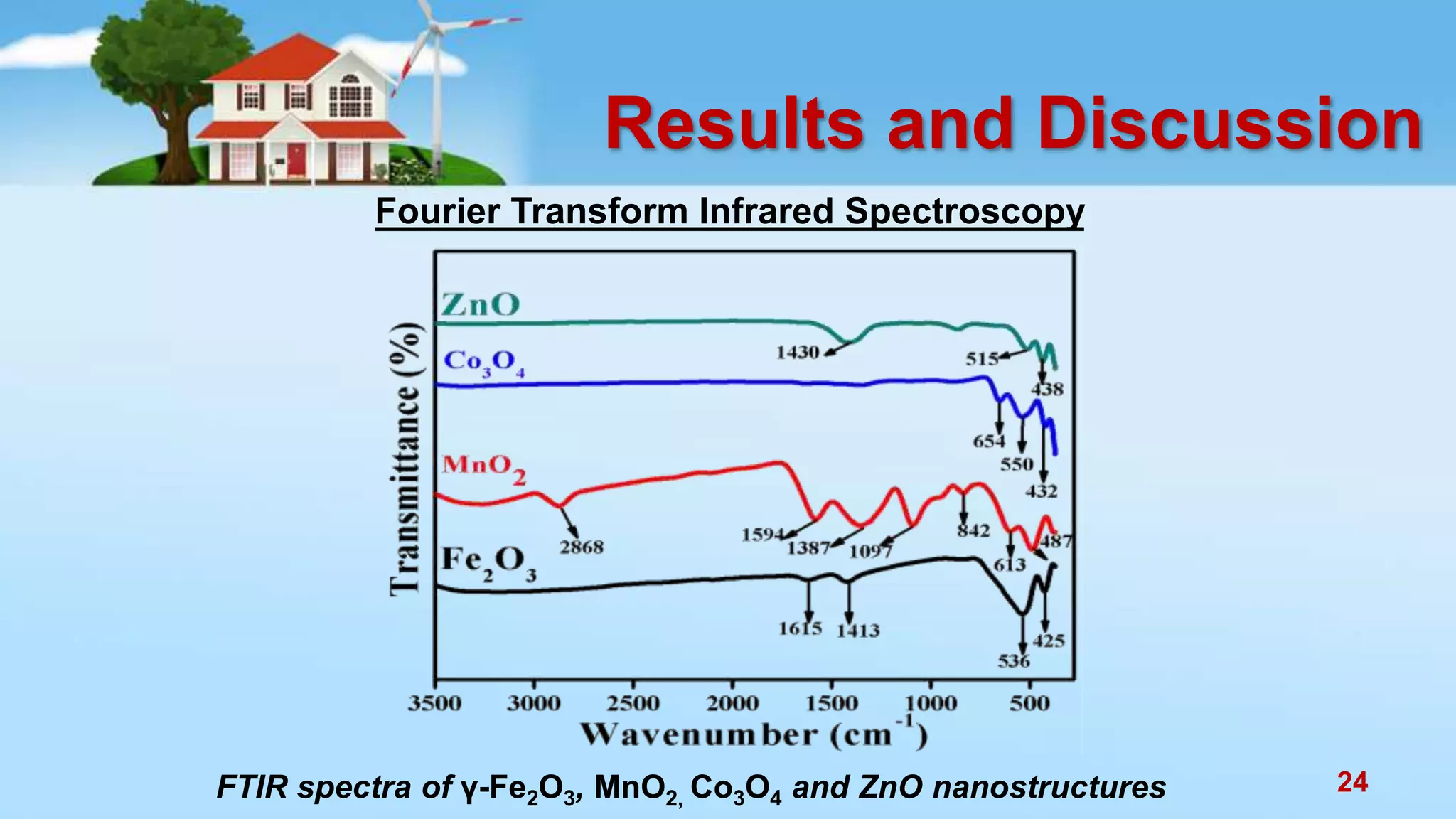
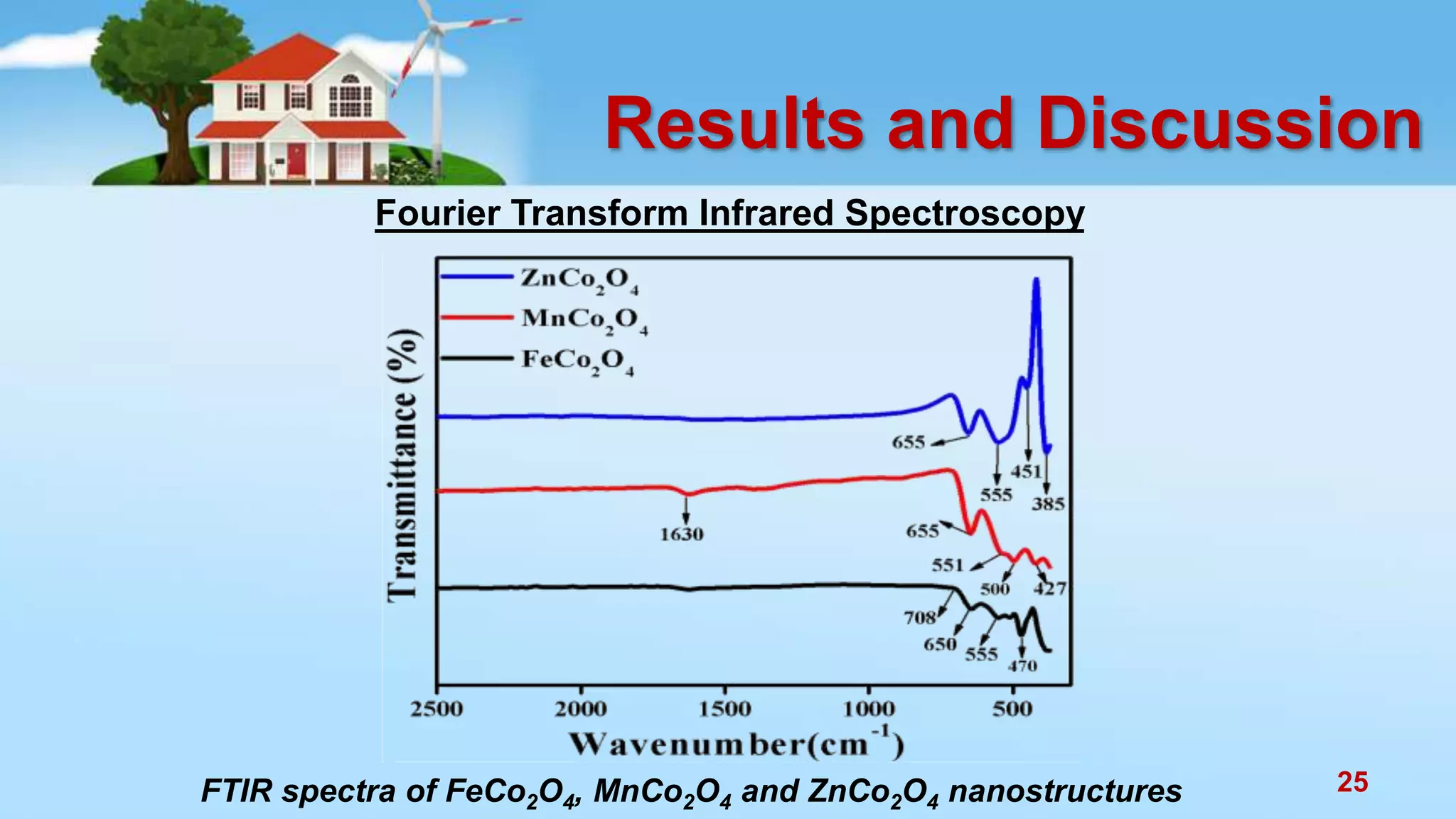
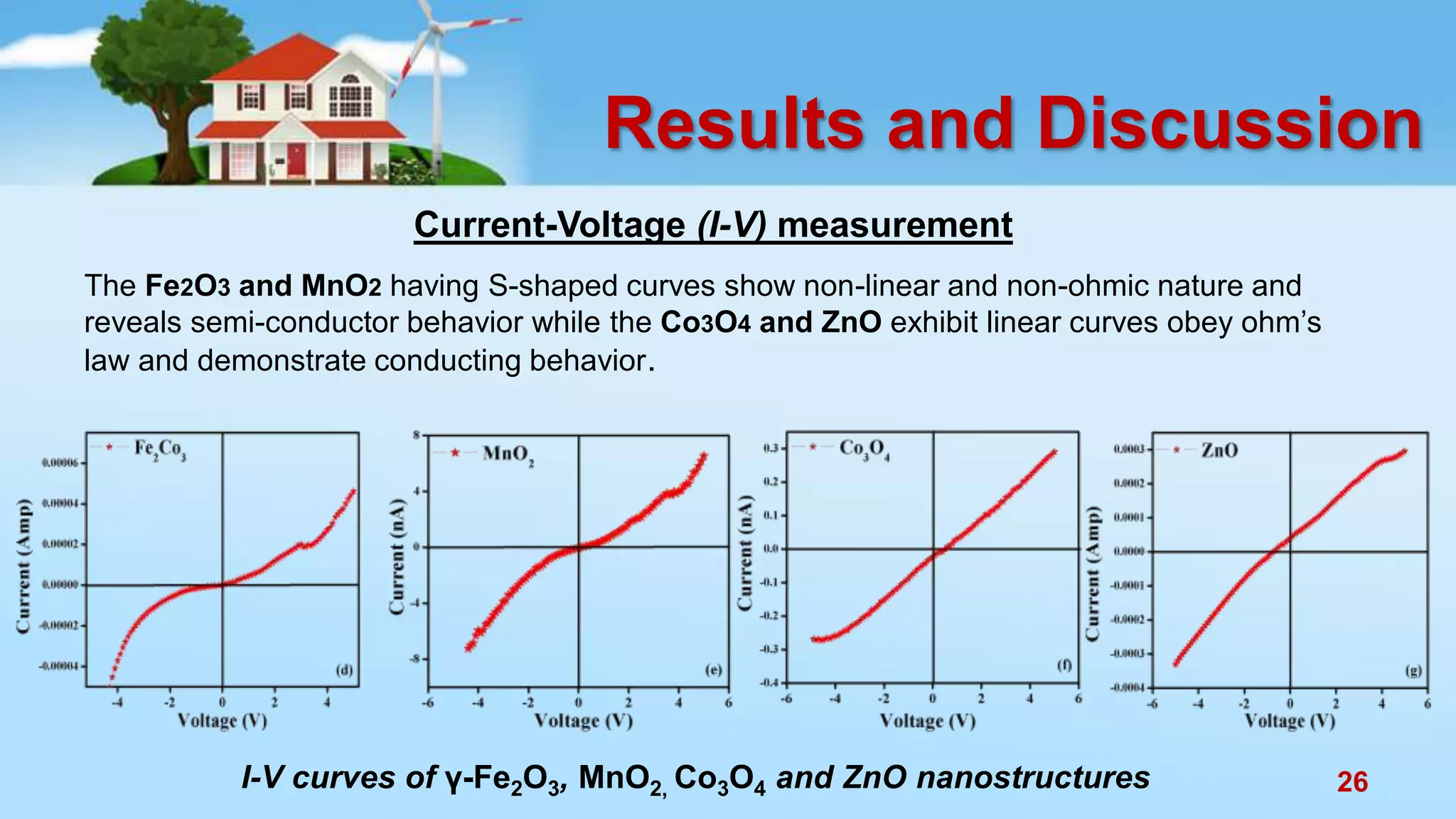
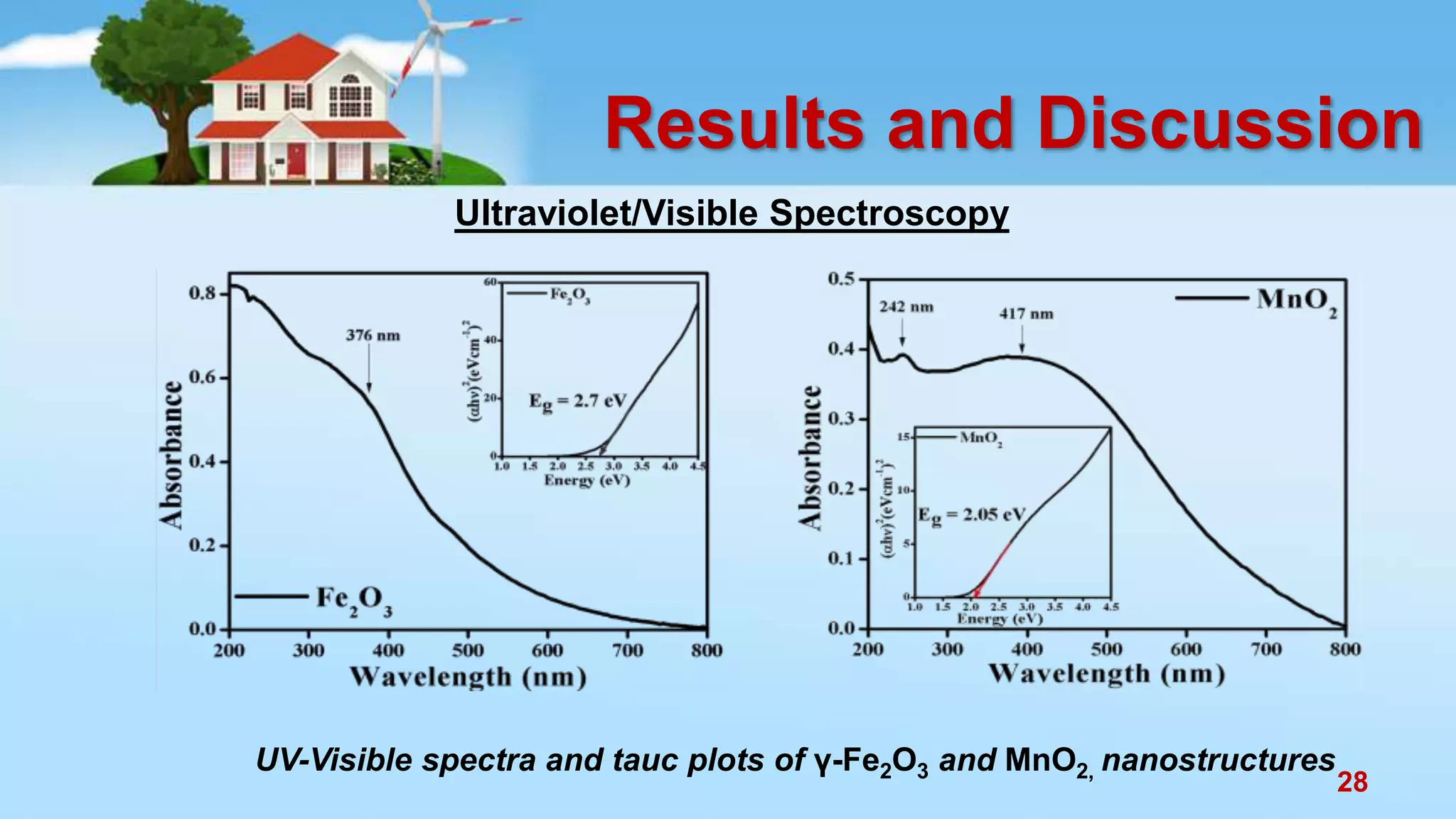
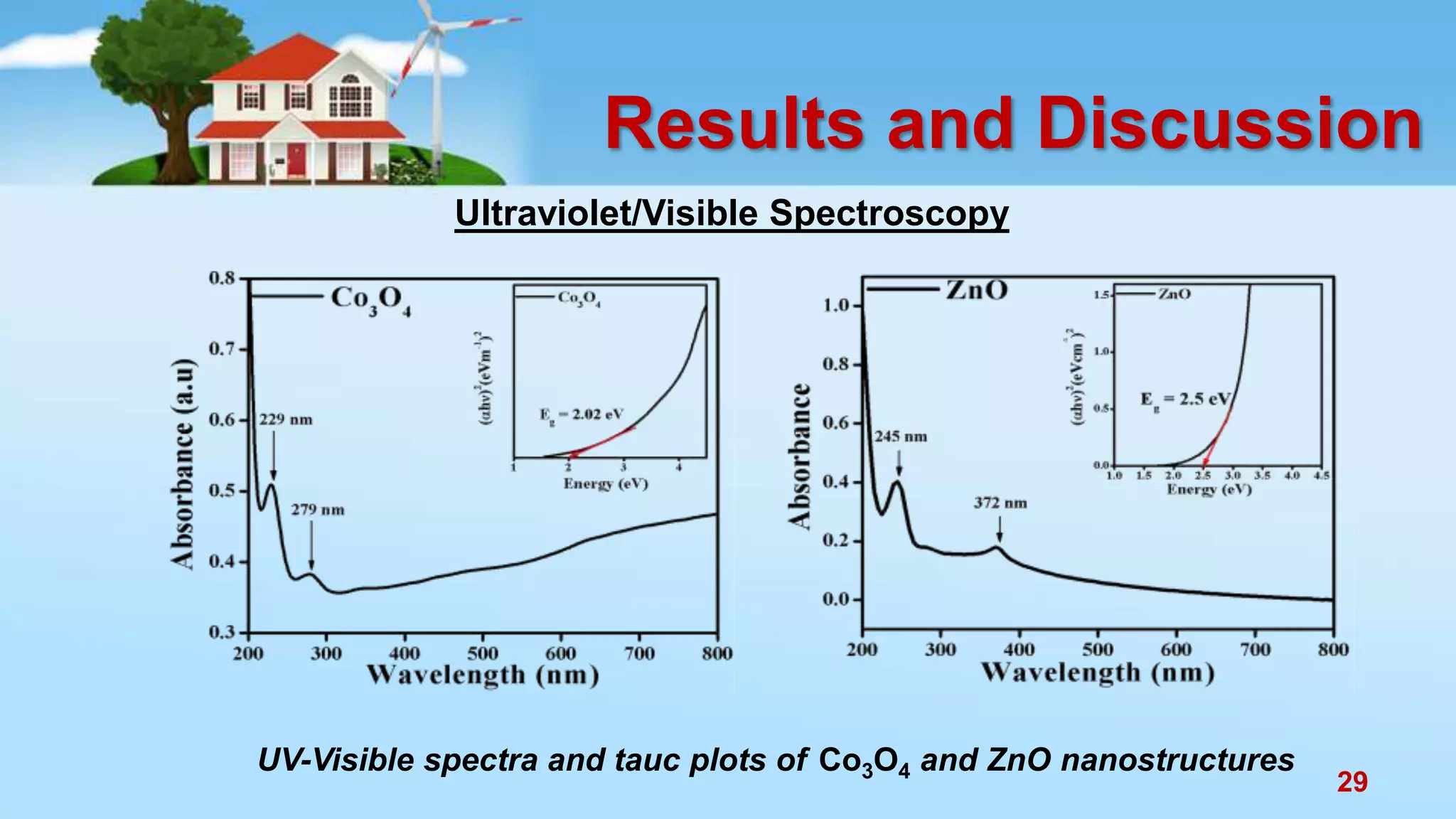
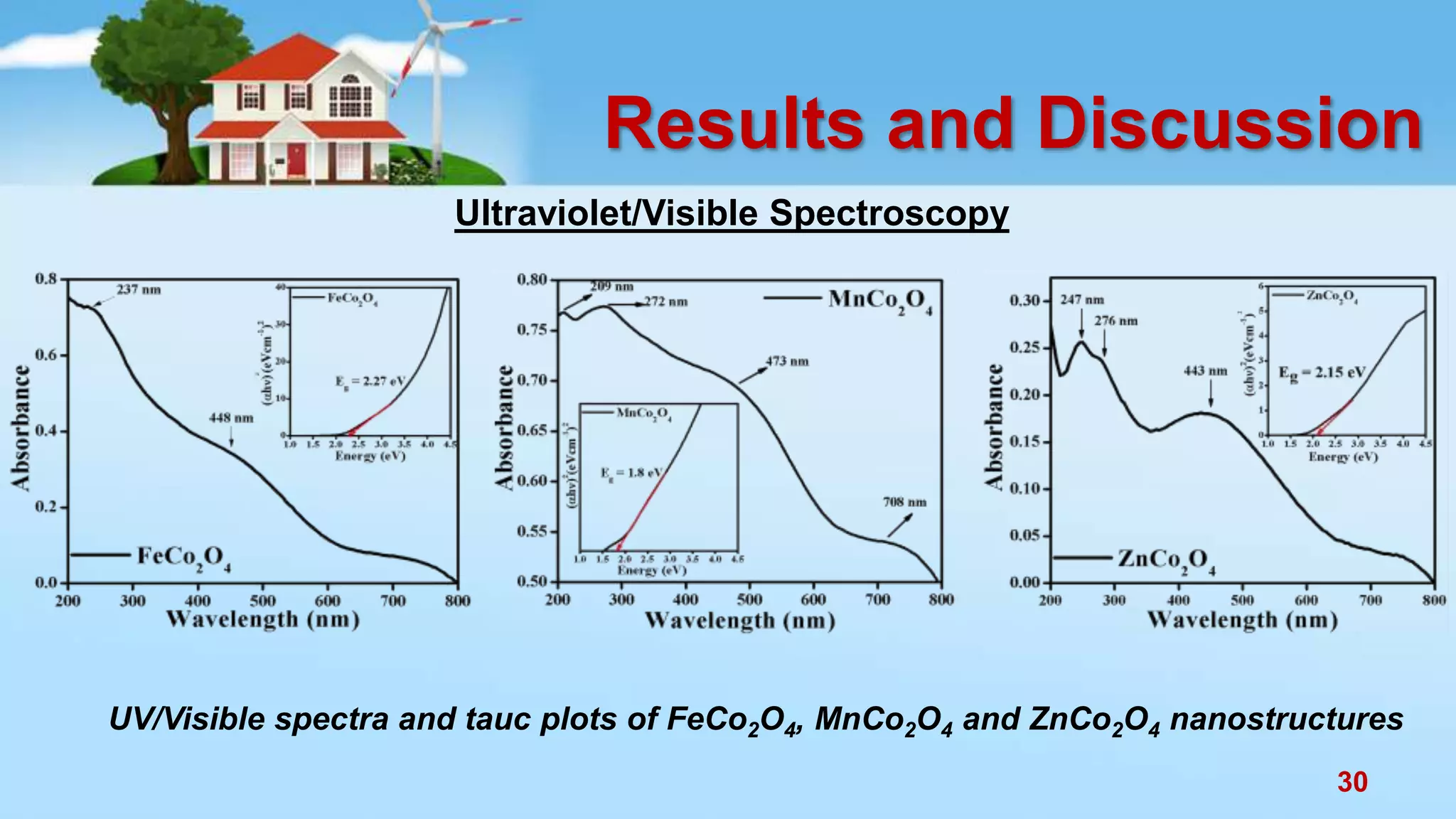
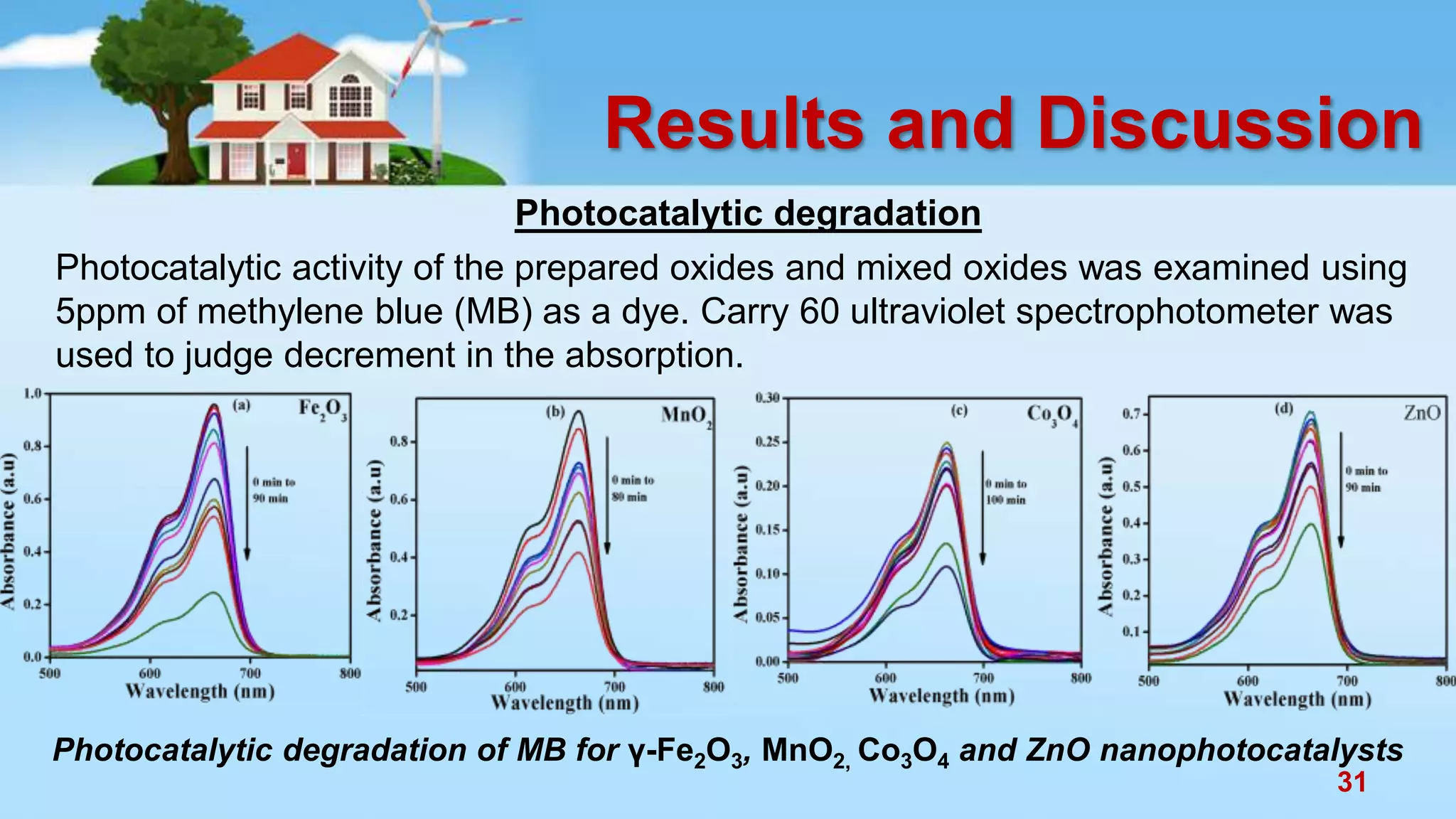
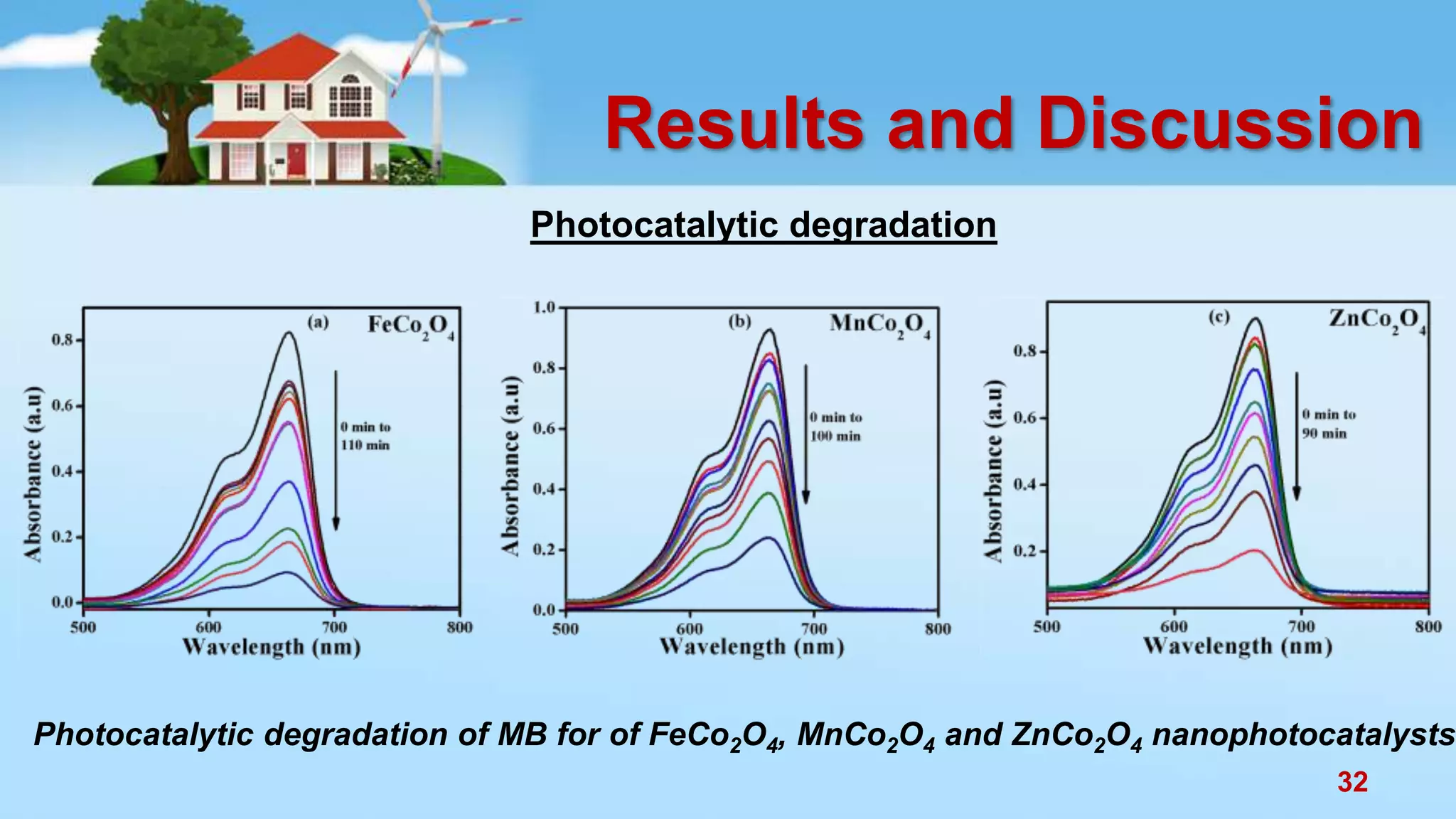
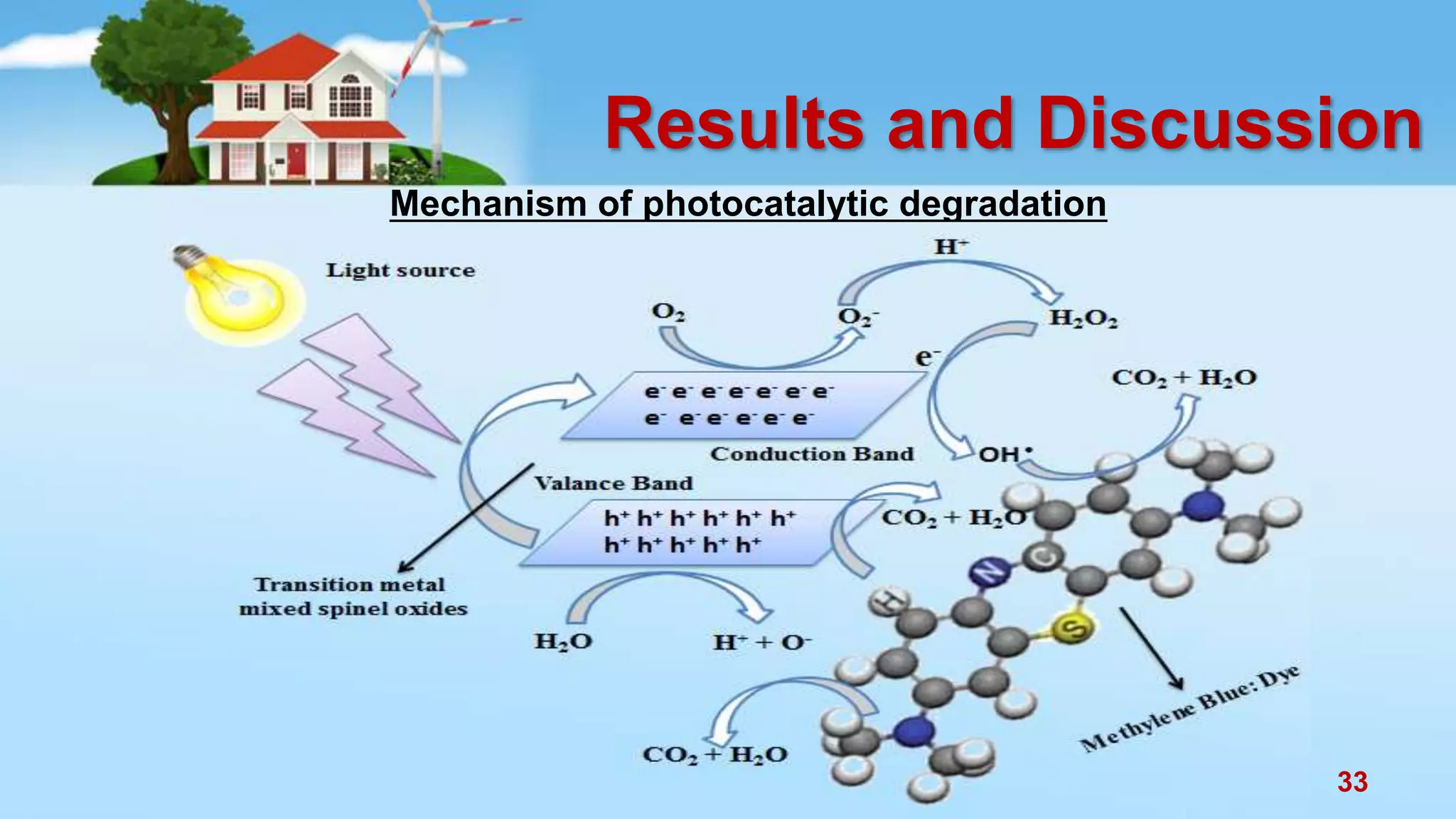
This document discusses the photocatalytic properties of transition metal oxides and their nanostructures synthesized through a wet chemical route. It explores various nanomaterials and their applications in photocatalysis, including experimental methods, results from different characterization techniques, and the comparative photocatalytic activity of spinel mixed oxides versus simple metal oxides. The findings indicate that spinel mixed oxides exhibit superior photocatalytic performance due to factors such as smaller crystallite size and increased surface area.