Embed presentation
Downloaded 50 times

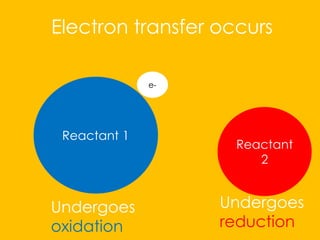



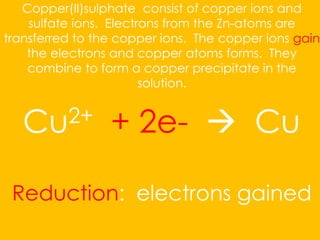



The document explains redox reactions, focusing on electron transfer between reactants. It details the oxidation of zinc, where zinc loses electrons to become positively charged, and the reduction of copper ions from copper(II) sulfate, which gain electrons to form solid copper. Spectator ions, such as sulfate, do not undergo any changes during the reaction.







