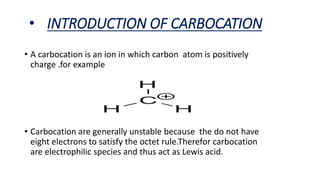
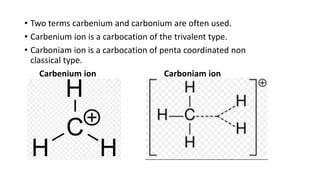
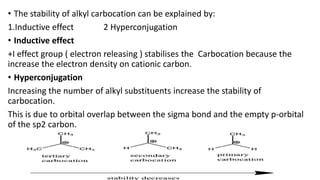
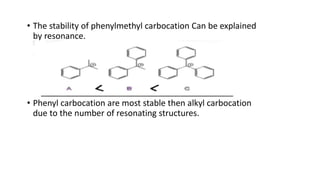
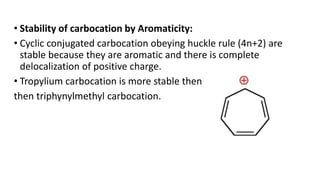
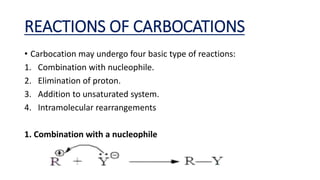
This document summarizes a student's report on carbocation reaction intermediates. It introduces carbocations as positively charged carbon species that are generally unstable. The document then covers:
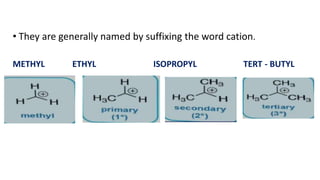
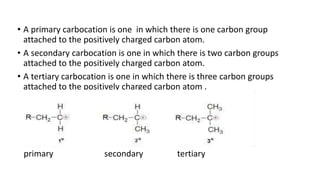
- Classification of primary, secondary, and tertiary carbocations
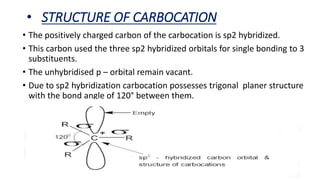
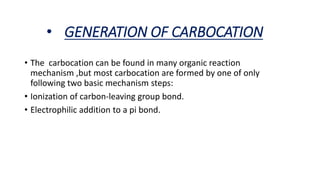
- Carbocation structure and generation mechanisms
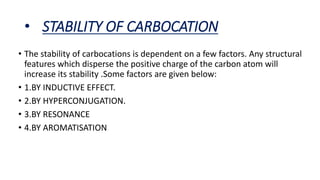
- Factors that influence carbocation stability such as inductive effects, hyperconjugation, and resonance
- Common reactions of carbocations including combination with nucleophiles, elimination of protons, addition to unsaturated systems, and intramolecular rearrangements.