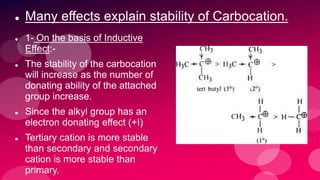
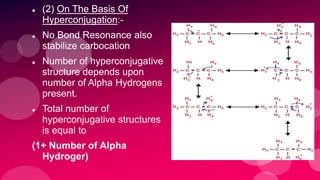
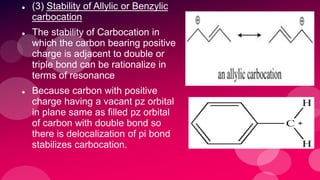
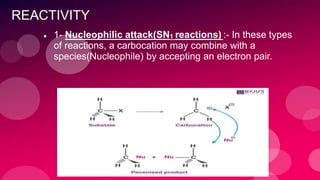
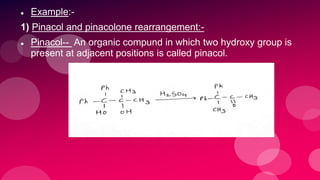
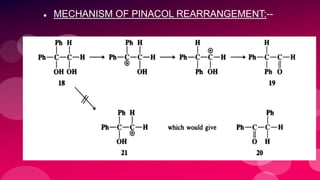
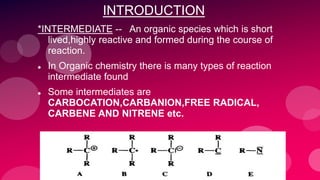
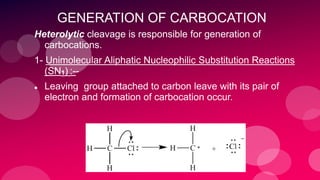
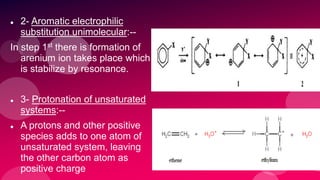
The document summarizes Naved Ahmad's seminar on carbocations, which are reactive intermediates formed through heterolytic cleavage. It discusses the generation, structure, stability and reactivity of carbocations, noting that tertiary carbocations are the most stable due to inductive and hyperconjugative effects. Examples of carbocation rearrangements like the pinacol and pinacolone rearrangements are provided to illustrate their reactivity.


![STRUCTURE
Carbon with positive
charge is sp2 hybridised.
Electronoic configuration of
carbon with positive charge
is [He]2s1 2px
1 2py
1 2pz
0
Bond angle is 1200.
Three sp2 hybrid orbitals
are in same plane.
Empty pz orbital at the
perpendicular to plane of
three hybrid orbitals.](https://image.slidesharecdn.com/navedseminar115324-240223052516-13037bfc/85/Reaction-intermediate-Carbocation-ppt-ppt-6-320.jpg)