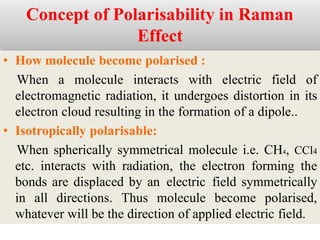
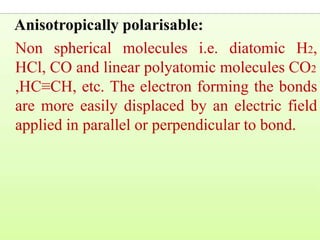
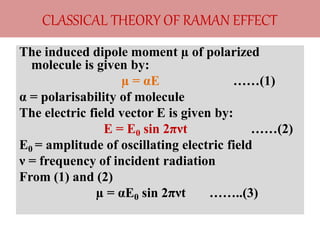
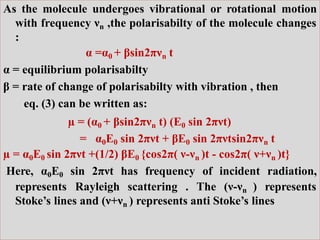
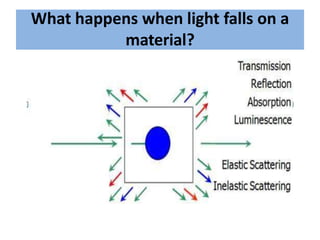
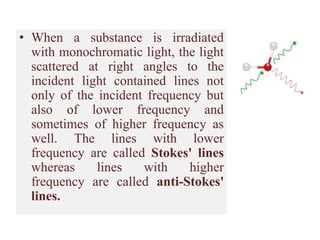
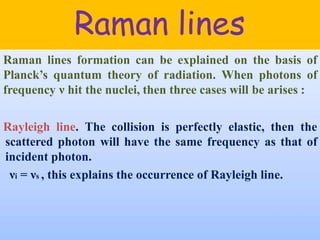
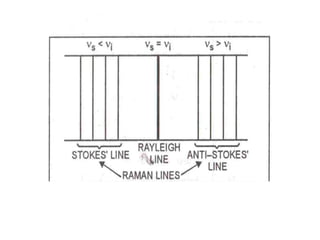
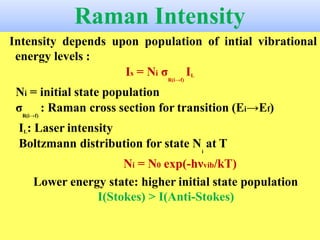
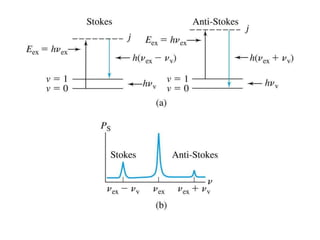
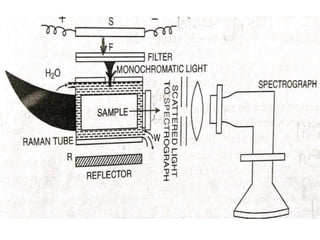
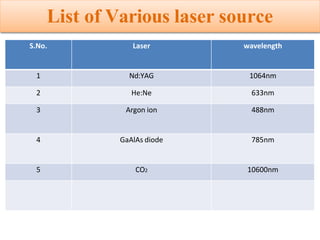
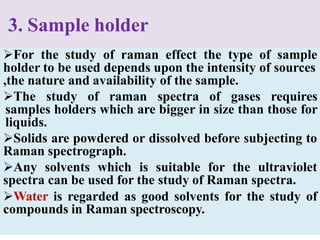
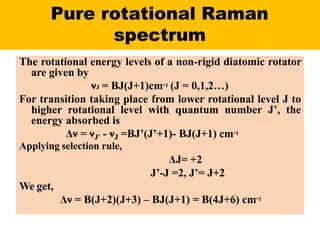
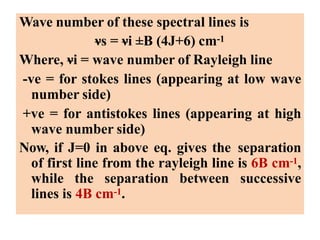
Okay, let me break this down step-by-step:
* HCl molecule is irradiated with mercury line of wavelength 434.8 nm
* To convert wavelength to wavenumber (cm-1):
Wavenumber (cm-1) = 10000/Wavelength (nm)
* So wavenumber of mercury line = 10000/434.8 = 2302.5 cm-1
* Fundamental vibrational frequency of H-Cl bond (ωe) = 34 cm-1
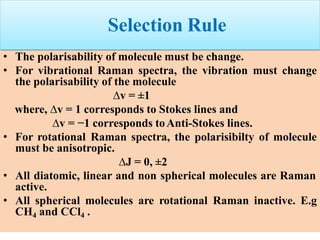
* Selection rule for Raman transition is Δv = ±1
* Stokes line = Incident wavenumber - Fundamental frequency
= 2302.5 - 34 = 2268.5 cm