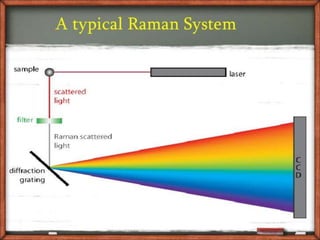
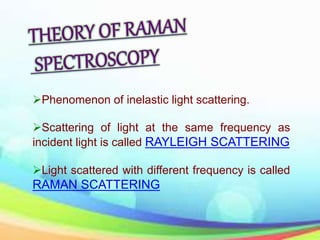
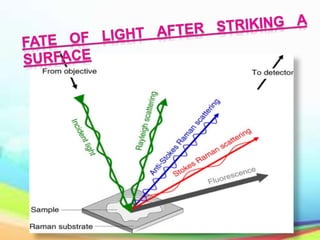
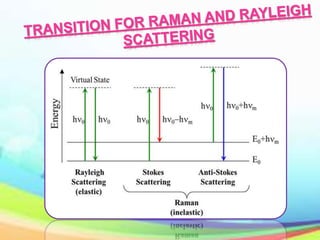
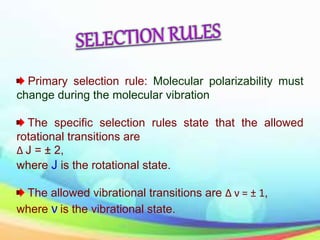
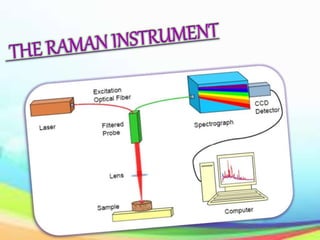
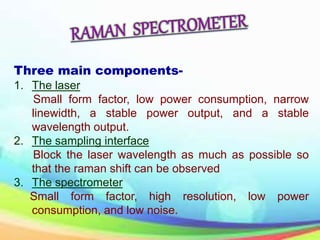
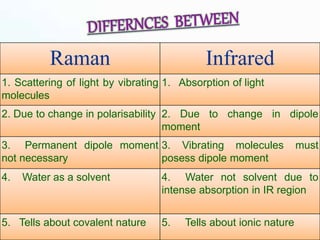
This document provides an overview of Raman spectroscopy. It discusses Raman scattering, which is the inelastic scattering of monochromatic light, usually from a laser, by molecules or atoms excited to higher vibrational or rotational energy levels. There are two types of Raman scattering: Stokes Raman scattering where the material absorbs energy and anti-Stokes Raman scattering where the material loses energy. Raman spectroscopy can be used to identify molecules and provide information about chemical bonds and molecular symmetry. It has various applications including medical use, detection of explosives, and investigation of historical documents.