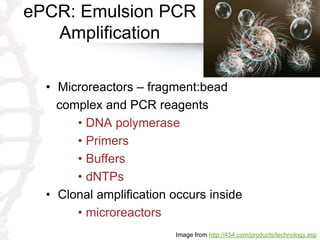
The document describes the process of 454-pyrosequencing for next generation sequencing. It involves the following main steps: 1) obtaining a sample and fragmenting its DNA library, 2) preparing the library with adapters and denaturing, 3) annealing fragments to beads in an emulsion to clonally amplify each fragment, 4) breaking the emulsion and loading beads onto a picotiter plate for sequencing, 5) performing pyrosequencing chemistry using enzymes and cameras to detect light signals from nucleotide washes to determine sequences. The data is then processed and analyzed for assembly, mapping, and variant detection.