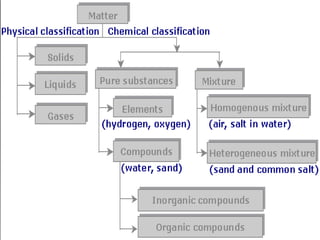
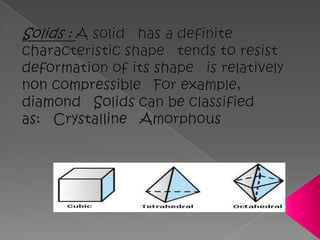
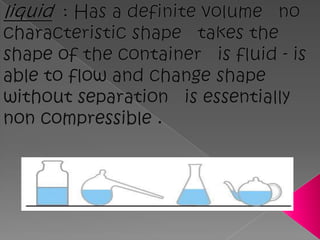
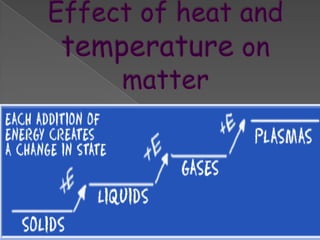
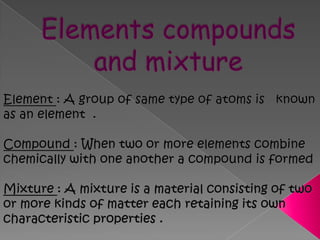
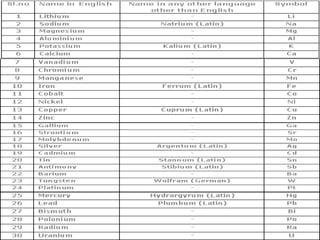
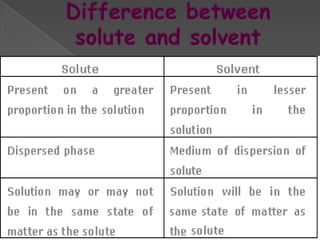
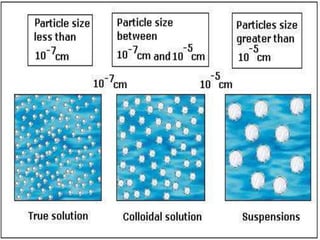
Matter exists in three states - solids, liquids, and gases. Solids have a definite shape and resist deformation, while liquids take the shape of their container and gases fill their vessel uniformly. Elements are made of only one type of atom, compounds are formed by chemical combination of elements, and mixtures maintain their individual properties. Dalton's atomic theory proposed that matter is made of indivisible atoms that combine in simple whole number ratios, though it was later found atoms have subatomic structure and isotopes with different masses. The mole concept allows quantifying particles on an atomic scale.