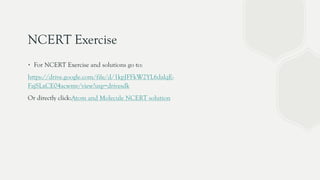
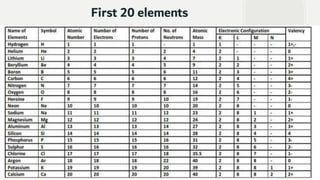
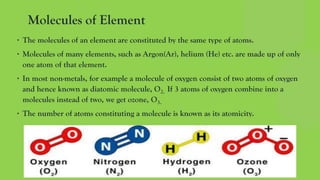
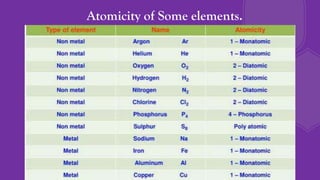
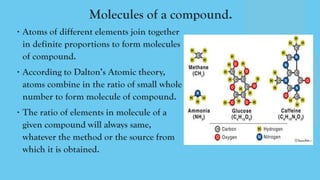
The document discusses the historical development of atomic theory, highlighting contributions from ancient philosophers like Maharishi Kanad, Democritus, and John Dalton. It explains key concepts such as the laws of chemical combination established by Lavoisier, Dalton's atomic theory, the nature and size of atoms, and the significance of chemical symbols and formulas in representing elements and compounds. Additionally, it covers the mole concept and the calculation of molecular and formula unit masses.
















![We will use brackets when two or mose
more of the same ions in the formula
Like, in the formula of ammonium
sulphate:
Symbol/Formula NH4 SO4
Charge 1+. 2–
Formula : [NH4]2SO4](https://image.slidesharecdn.com/atomsandmoleculesbyraxitgupta-211110093035/85/Atoms-and-molecules-class-9-27-320.jpg)