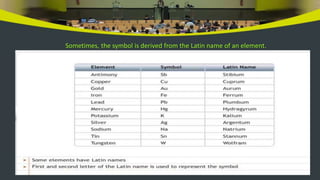
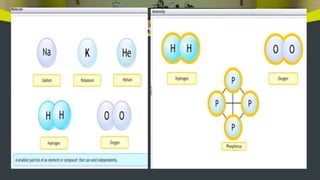
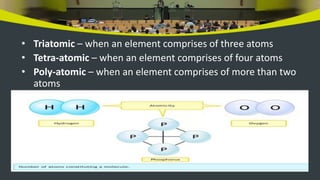
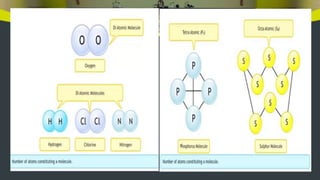
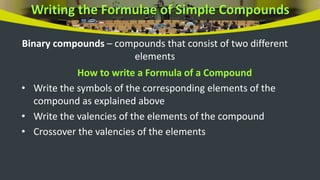
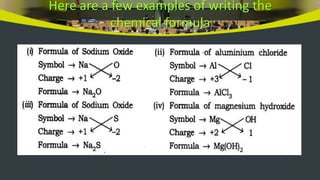
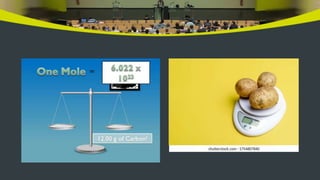
This document provides an overview of atoms and molecules. It defines key terms like atom, molecule, ion, and discusses Dalton's atomic theory and its postulates. The document explains that atoms are the smallest particles that make up matter and combine to form molecules or ions. It discusses how elements are represented by symbols and how atomic mass is measured in atomic mass units relative to carbon-12. The document also summarizes laws of chemical combination and provides examples of writing chemical formulas based on valencies of elements.