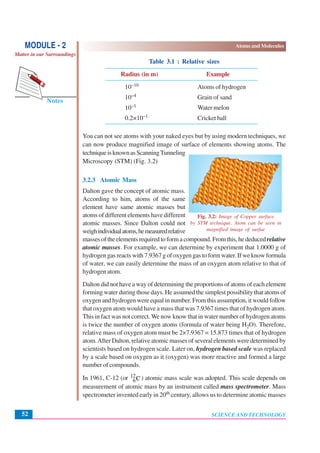
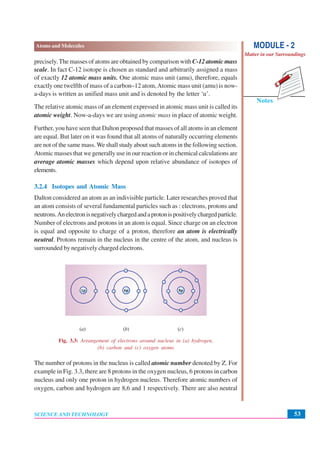
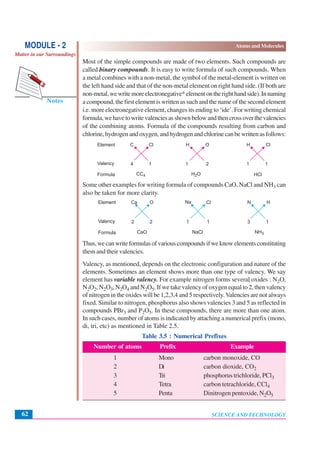
This document discusses Dalton's atomic theory from the early 1800s. It introduces some key ideas:
1) Dalton proposed that matter is made of indivisible atoms and that atoms of different elements have different masses.
2) His theory explained laws of conservation of mass and constant proportions from chemical experiments.
3) It introduced ideas that in compounds, elements combine in small whole number ratios and that during chemical reactions atoms are rearranged but not destroyed.