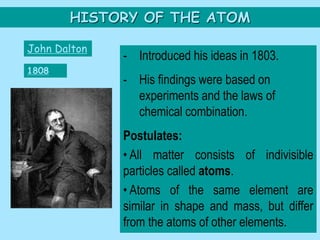
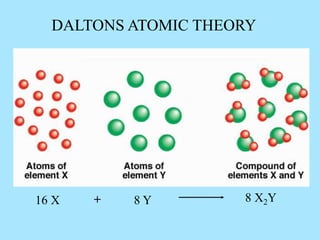
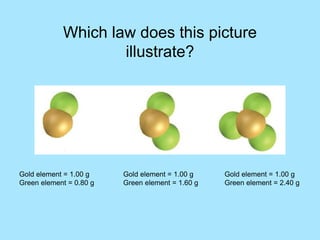
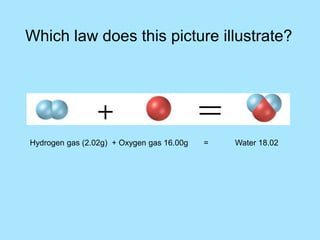
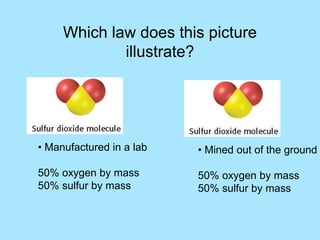
This picture illustrates the Law of Definite Proportions. The law states that a given compound always contains the same elements in a fixed ratio by mass. Here, both samples of sulfur dioxide contain oxygen and sulfur in a 50:50 ratio by mass, regardless of whether it was manufactured in a lab or mined from the ground.