This document provides a summary of basic chemistry concepts covered in chapters 2 and 3 of a unit 1 notes document. It discusses the following key points in 3 sentences:
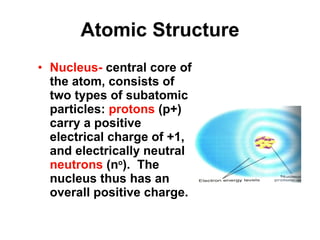
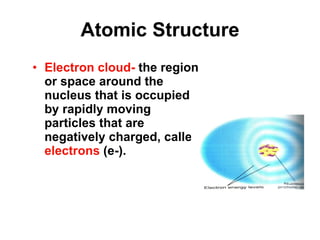
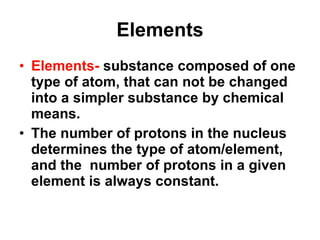
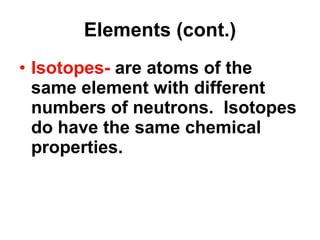
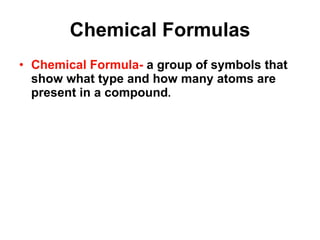
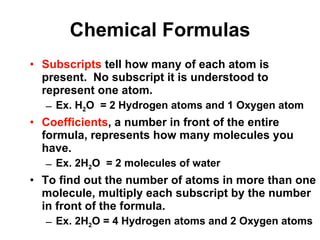
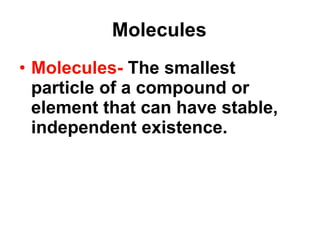
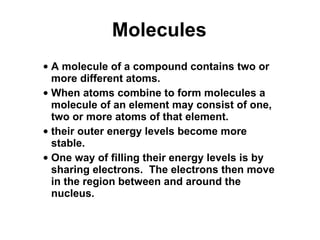
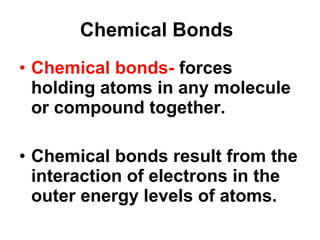
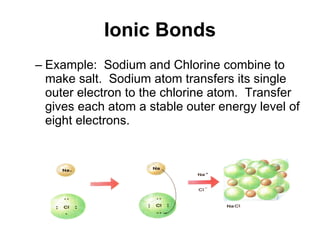
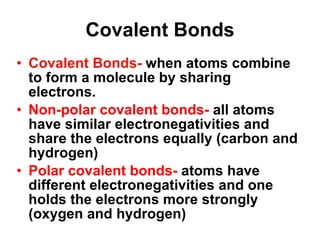
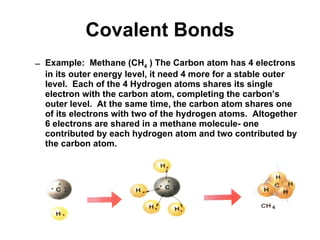
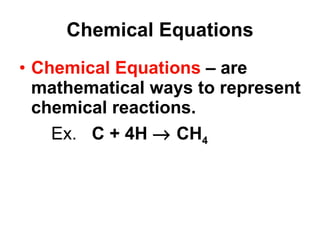
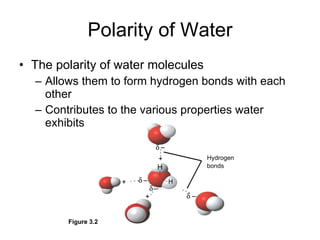
Matter is anything that takes up space and has specific physical and chemical properties. Atoms are the basic building blocks of matter and consist of a nucleus with protons and neutrons surrounded by electrons. Compounds are formed when two or more elements chemically combine, resulting in new substances with different properties than the individual elements.





















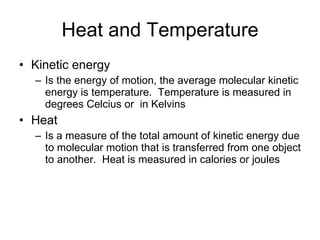







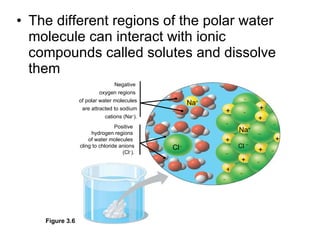
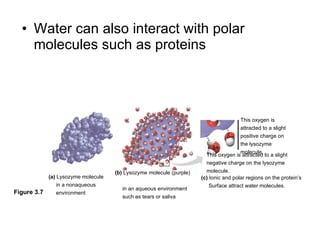



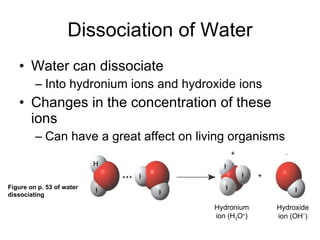

![The pH scale and pH values of various aqueous solutions Increasingly Acidic [H + ] > [OH – ] Increasingly Basic [H + ] < [OH – ] Neutral [H + ] = [OH – ] Oven cleaner 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 pH Scale Battery acid Digestive (stomach) juice, lemon juice Vinegar, beer, wine, cola Tomato juice Black coffee Rainwater Urine Pure water Human blood Seawater Milk of magnesia Household ammonia Household bleach Figure 3.8](https://image.slidesharecdn.com/23-blahblahbalh-100902181731-phpapp01/85/Chapters-2-3-57-320.jpg)