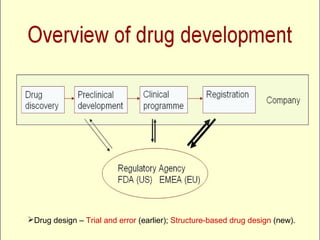
Drug development involves basic research to identify drug targets and applied research to develop treatments. Preclinical trials test drug safety and efficacy in animals prior to human trials. They involve pharmacokinetic, pharmacodynamic and toxicology studies in two animal species. This provides data on effective and toxic doses, screens the drug's activity, and identifies formulation. Preclinical trials help determine if a drug warrants further development or should be terminated. They aim to predict potential human adverse effects and provide guidance for initial human clinical trials. However, extrapolating animal data to humans has limitations due to interspecies differences.





![Introduction (contd.)
Scope of Preclinical studies:
• Preliminary evaluation of the p’cology, p’cokinetics, p’codynamics, dose–
response profiles, safety and toxicological potential of a drug.
• Analyze physicochemical characteristics of the test compound.
• Determines the optimal formulation and dose for phase I clinical trials.
• Provides the rationale for the proposed therapeutic indication.
• Preclinical p’cological and animal toxicity results from studies →
Approval or disapproval to initiate human clinical trials.
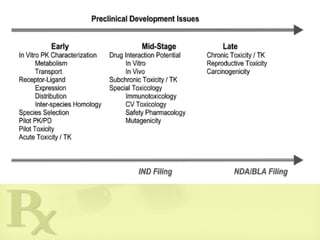
Major tests performed on a drug candidate during preclinical trials :
• PK and PD studies
• Bioequivalence and bioavailability
• Toxicity studies [Acute and chronic toxicity; Mutagenicity or
carcinogenicity; Immunotoxicity and other tests].](https://image.slidesharecdn.com/preclinicaltesting-anintro-150928035035-lva1-app6892/85/Preclinical-testing-An-intro-8-320.jpg)



![ Similar or variable drug metabolism between species (based on a
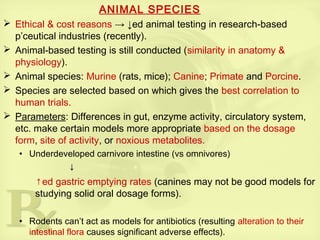
drugs’ functional groups) → affects efficacy and toxicology.
Larger species (dogs, pigs, sheep) are usually used for most tests
(similar-sized model as that of a human).
Similarity in specific organs or organ system physiology
• swine (dermatological & coronary stent studies)
• goats (mammary implant studies)
• dogs (gastric studies).
Pre-clinical trials enable in establishing the ‘No Observable Effect
Levels’ (NOEL) / ‘No Observable After Effect Levels’ (NOAELs) on
drugs.
NOEL / NOAEL
• Indicates the optimal initial phase I clinical trial dosage levels on a
mass API per mass patient basis.
• Achieving the highest drug exposure levels (systemic / local) with the
lowest dosage administered.
[API – Active Pharmaceutical Ingredient]](https://image.slidesharecdn.com/preclinicaltesting-anintro-150928035035-lva1-app6892/85/Preclinical-testing-An-intro-14-320.jpg)



![Skin
Little value in skin irritancy testing (enormous variability in the skin
response of different animal species to toxic chemicals).
If animal dermal tolerance testing is justified,
1] Single-dose dermal tolerance test
• in rabbits (on shaved intact skin and shaved abraded skin).
• examine exposed skin for erythema, edema, desquamation, scab
formation and other lesions.
• note changes at 24, 48 and 72 hours.
• follow up (upto 8 days) may be necessary.
2] Repeated-dose dermal tolerance test
• also done in rabbits (intact and abraded shaved skin);
• follow-up for periods of upto 4 weeks.](https://image.slidesharecdn.com/preclinicaltesting-anintro-150928035035-lva1-app6892/85/Preclinical-testing-An-intro-18-320.jpg)
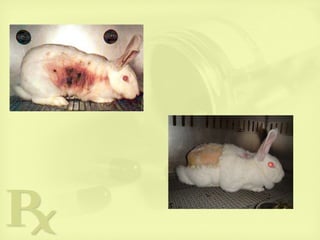

![Eyes
Any chemical with irritant or corrosive properties when
applied to the skin can irritate the cornea and conjunctiva
Draize test in rabbits: most widely used test for prediction
of ophthalmological irritancy; it is injurious to the
experimental animals.
• Local ocular toxicity and tolerance testing – ophthalmic
products (topical) or products applied close to the eye [face
or hair (medicated shampoos)],
• single administration (in rabbit).
• also examine for anesthetizing properties.
• evaluation of the eyes, lids, conjunctivae, nictitating
membrane, cornea, and iris in one eye is necessary; other
eye (control).
Repeat ocular dosing:
• daily administration in the rabbit for not more than 4 weeks.
• based on the results of the acute, single-dose study.](https://image.slidesharecdn.com/preclinicaltesting-anintro-150928035035-lva1-app6892/85/Preclinical-testing-An-intro-21-320.jpg)

![Value of the LD50 Test
Twofold:
1] To determine the Therapeutic margin (margin between
effective and toxic doses).
2] Comparing lethal effects with blood levels of the active
principle.
Pointers
Always consider in conjunction with other relevant
information.
Conducting the LD50 test on large animals should be
discontinued.
Conduct the test on a limited number of small animals, w/
detailed recording of symptoms and pathology.
LD50 test should not be conducted with p’cologically inert
subs. [max. of 5g/kg (oral admn.); 2g/kg (parenteral) is
sufficient, if death or acute symptoms are not produced].
Don’t conduct in newborn animals.](https://image.slidesharecdn.com/preclinicaltesting-anintro-150928035035-lva1-app6892/85/Preclinical-testing-An-intro-23-320.jpg)







