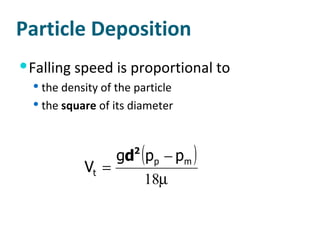
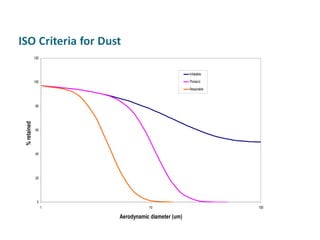
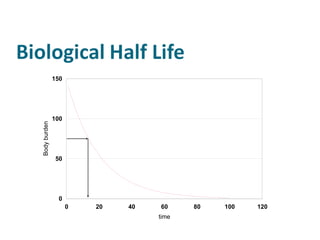
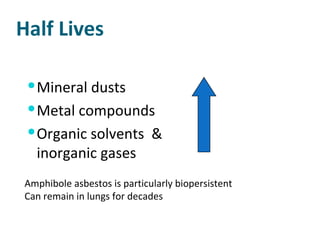
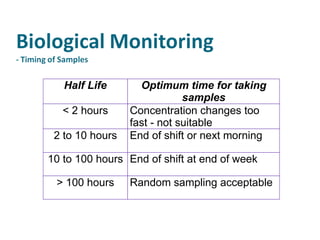
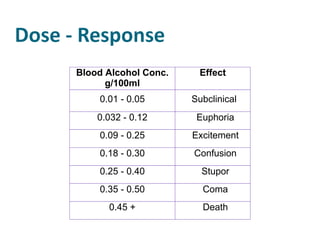
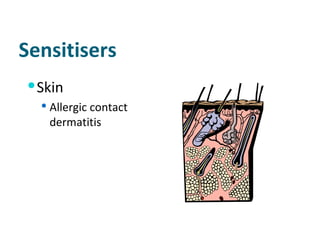
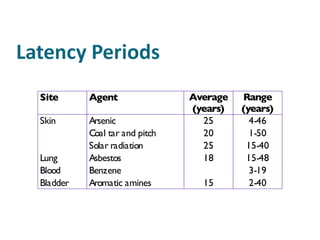
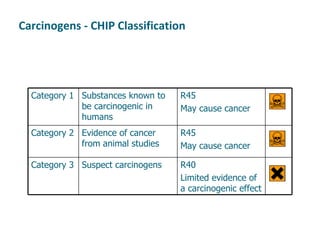
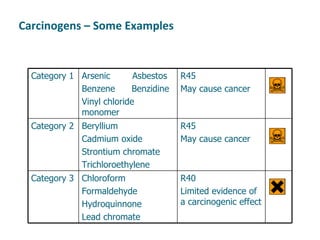
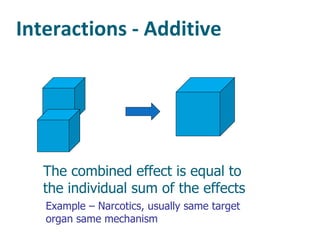
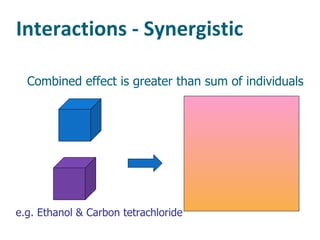
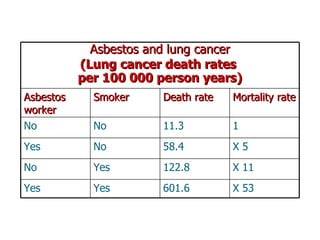
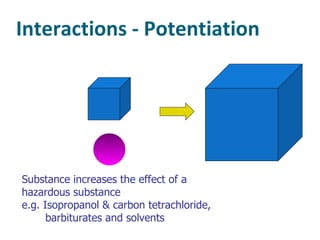
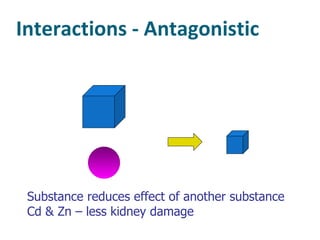
The document discusses various topics related to toxicology including:
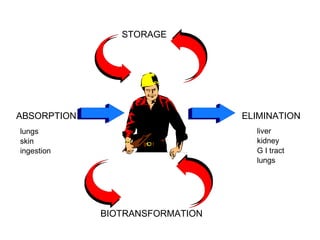
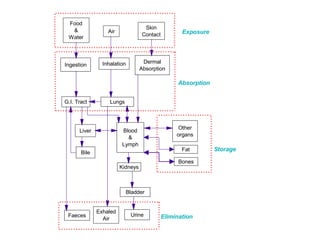
- Absorption, distribution, metabolism and excretion of chemicals in the body
- Factors that affect absorption such as routes of entry and chemical properties
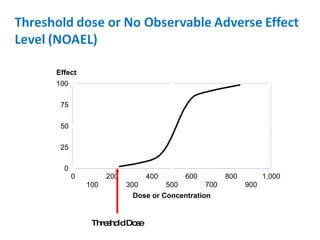
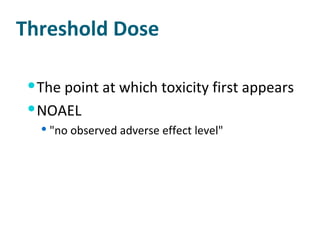
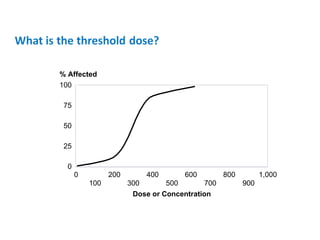
- Threshold doses and variability in individual susceptibility
- Acute and chronic effects of toxins
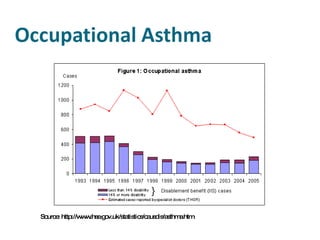
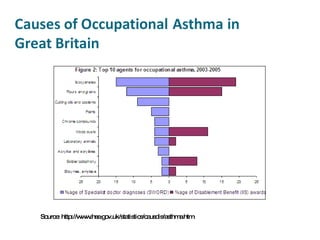
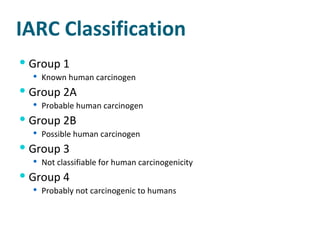
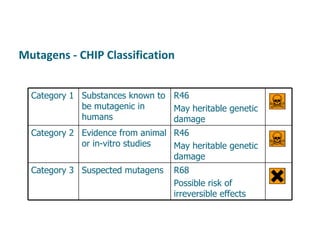
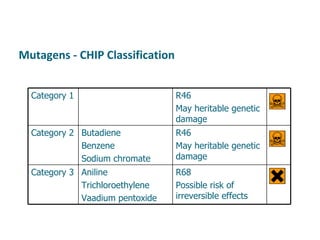
- Carcinogens and mutagens and their classification
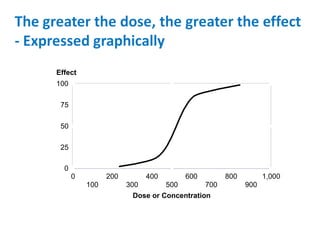
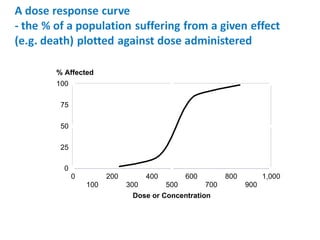
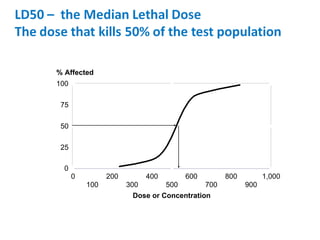
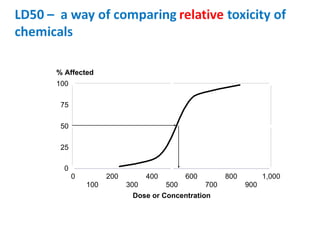
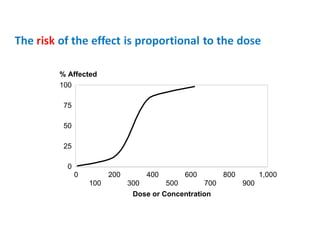
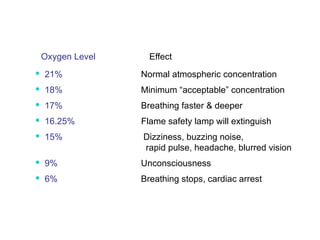
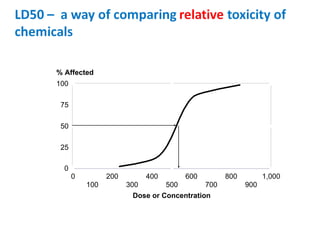
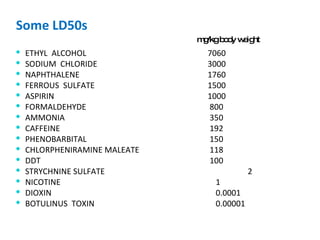
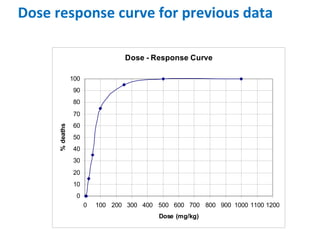
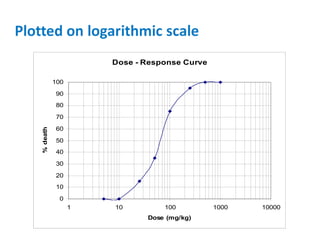
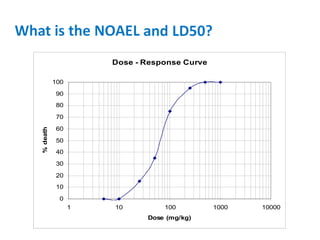
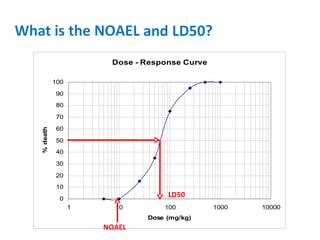
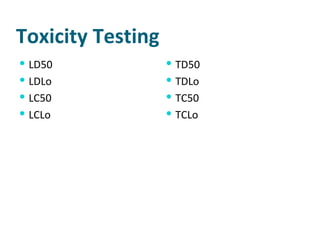
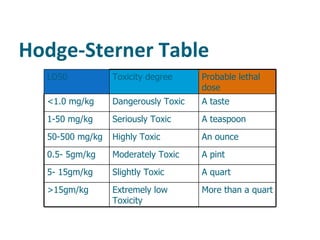
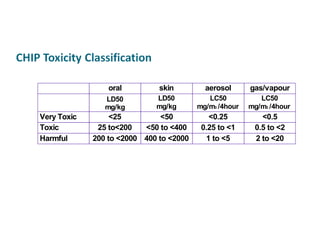
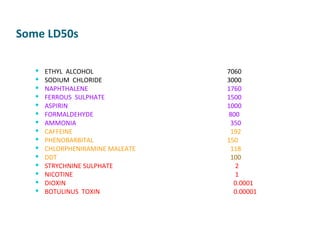
- Dose-response relationships and metrics like LD50
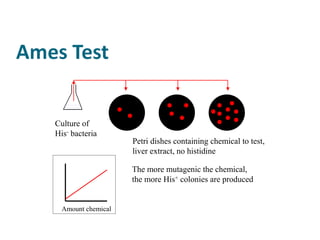
- Toxicity testing methods including the Ames test