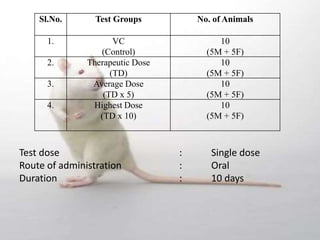
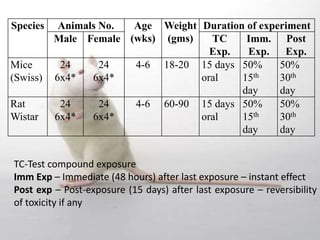
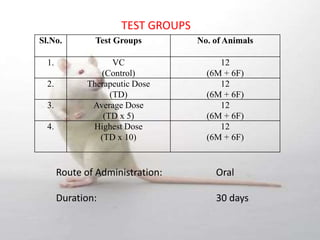
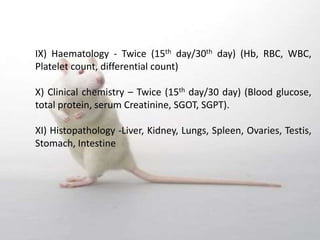
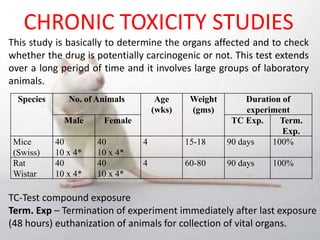
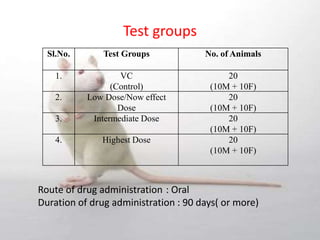
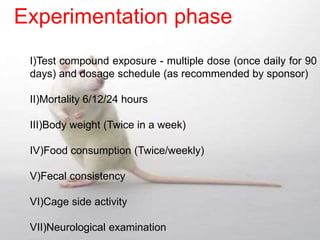
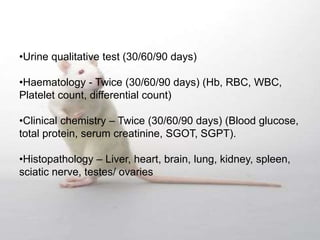
The document provides information about toxicity studies conducted to evaluate the safety of plant drugs used in Ayurveda and Siddha medicine. It discusses the various types of toxicity studies including acute, sub-acute, chronic, and reproductive toxicity studies. Key aspects covered are the study designs, animals used, dosages, observations made and parameters evaluated to understand the adverse effects of test substances. Guidelines are provided for conducting toxicity studies and evaluating the results to determine therapeutic indices and predict safety for clinical use.





























![Up‐and‐Down Procedure (OECDTG 425)
•The main test consists of a single ordered dose progression in which
animals are dosed , one at a time , at a minimum of 48-hour intervals.
• The first animal receives a dose a step below the level of the best
estimate of the LD50 . If the animal survives, the dose for the next
animal is increased by [a factor of] 3.2 times the original dose ; if it
dies, the dose for the next animal is decreased by a similar dose
progression.
•Dosing is stopped when
• 3 consecutive animals survive at the upper bound;
• 5 reversals occur in any 6 consecutive animals tested
• LD 50 is calculated.](https://image.slidesharecdn.com/tocixitystudies-161012210535/85/Tocixity-studies-40-320.jpg)



