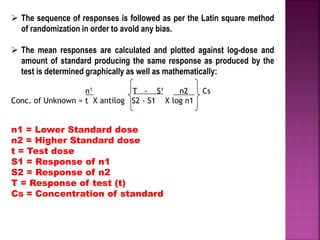
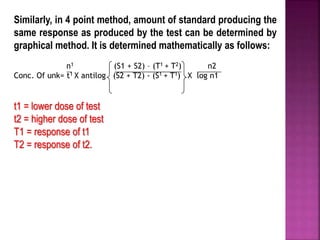
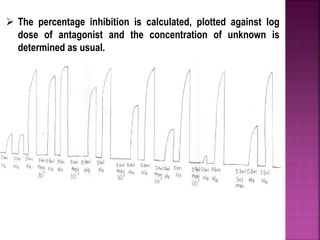
This document discusses bioassay methods for quantifying the potency and concentration of drugs. It defines bioassay as using biological methods to estimate the potency of an active drug principle. Various types of bioassays are described, including quantal and graded response assays. Specific methods covered include end-point, matching and bracketing, graphical, and multiple point assays. Examples of bioassays discussed include assays for digitalis, d-tubocurarine, oxytocin, and histamine.