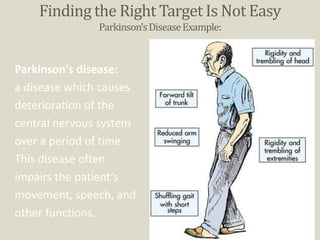
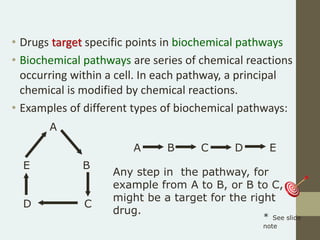
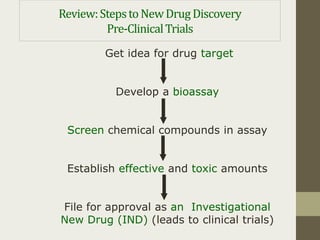
Preclinical trials involve testing potential new drugs on animals before human testing to determine if they are safe and effective. This process involves several key steps: 1) Researchers identify a biological target related to a disease through basic research. 2) They develop a bioassay using cells or animals to test drug effects. 3) Potential drug compounds are screened in the bioassay to see if they act on the target. 4) Effective and toxic doses are established to find a safe range. If successful, 5) approval is sought from the FDA to begin clinical trials in humans.