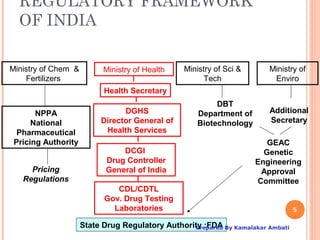
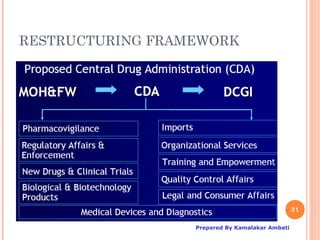
The document outlines the regulatory framework for clinical trials in India, specifically focusing on Schedule Y, which guides the permission process for importing and manufacturing new drugs and conducting clinical trials. It highlights the roles of the Drugs Controller General of India (DCGI), the organizational structure, and the requirements for clinical trial applications, including ethics committee approvals and classifications of trials. Additionally, the document discusses proposed changes made to Schedule Y from its 1985 version to the amended 2005 version, emphasizing the need for stringent protocols and monitoring post-marketing for drug safety.