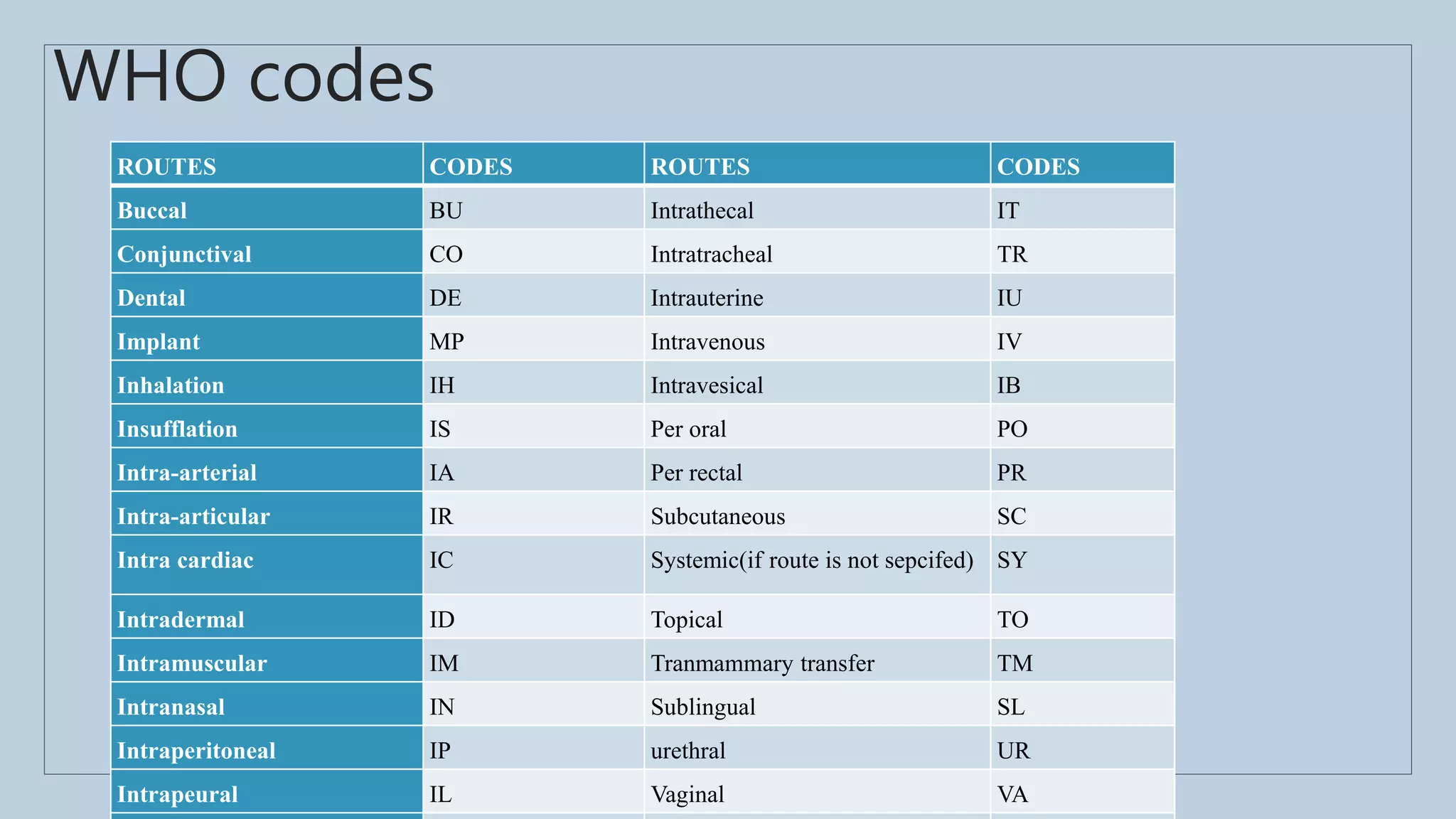
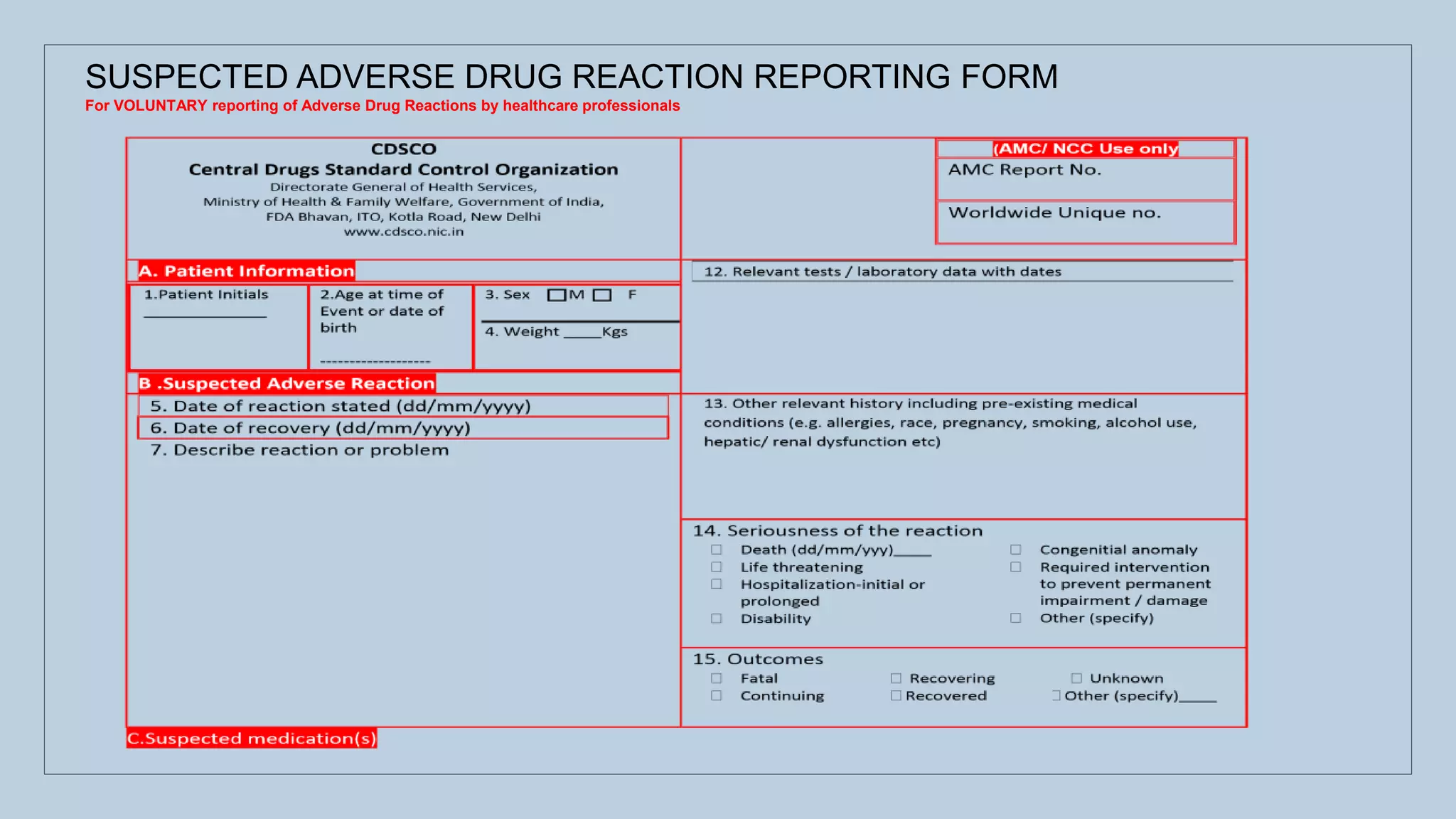
The document outlines the spontaneous adverse drug reaction (ADR) reporting system in India, emphasizing the roles of health professionals and pharmaceutical companies in reporting suspected ADRs to regulatory authorities. It details the process of data acquisition, assessment, and interpretation, alongside specific reporting requirements and forms used in different countries. Additionally, it describes the infrastructure for ADR monitoring, including various centers and tools implemented to enhance reporting and improve pharmacovigilance in the country.