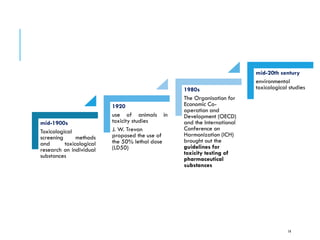
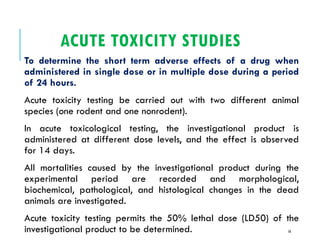
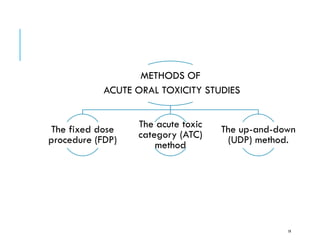
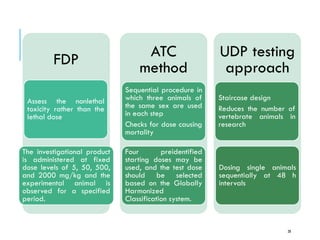
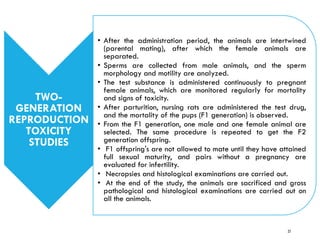
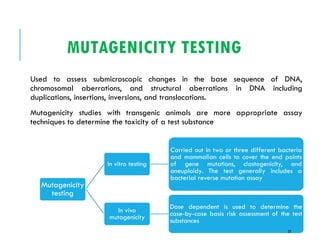
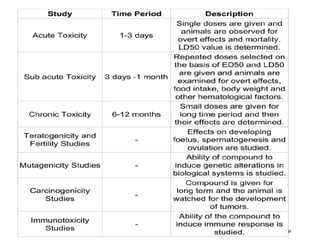
Pre-clinical screening involves testing potential new drugs in animal models before human trials to evaluate safety and efficacy. This includes pharmacological screening to determine mechanism of action and dose response, as well as toxicological testing to identify adverse effects and calculate safe starting doses for clinical trials. Studies progress from molecular and cellular assays to whole animal experiments. Acute and repeated dose toxicity tests are followed by sub-chronic and chronic studies to identify long-term effects. These pre-clinical studies aim to generate data required to deem a new compound safe enough for initial human testing.