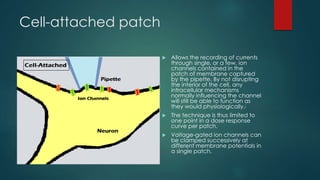
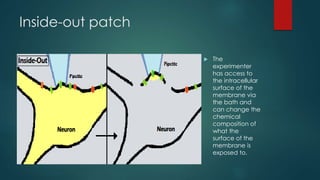
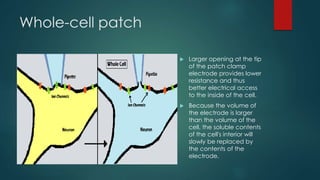
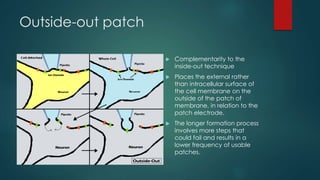
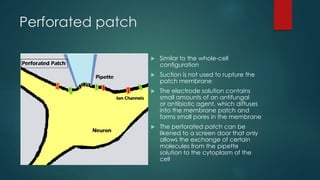
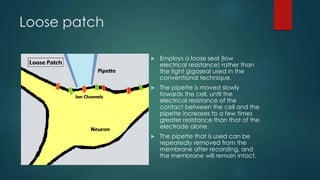
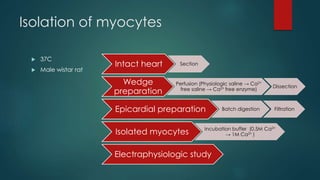
The patch-clamp technique allows the study of single ion channels in cells. It was developed in the late 1970s and early 1980s by Erwin Neher and Bert Sakmann, who received the Nobel Prize for this work. There are different variations of the patch-clamp technique that provide access to the inside or outside of the cell membrane to study channel properties under various conditions. The technique uses a glass pipette pressed against a cell to form a high resistance seal and then precisely measures electric currents flowing through individual or multiple ion channels.