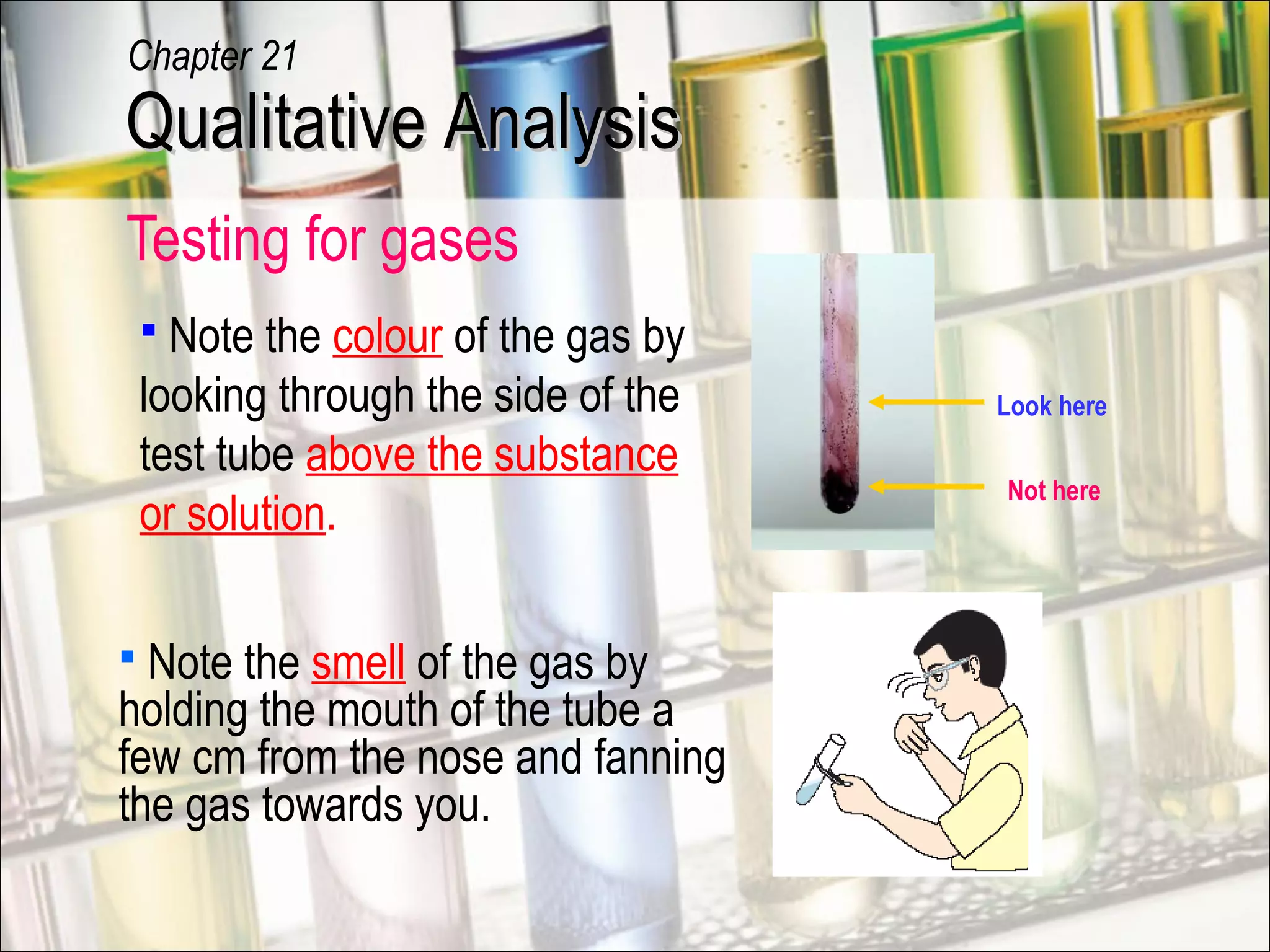
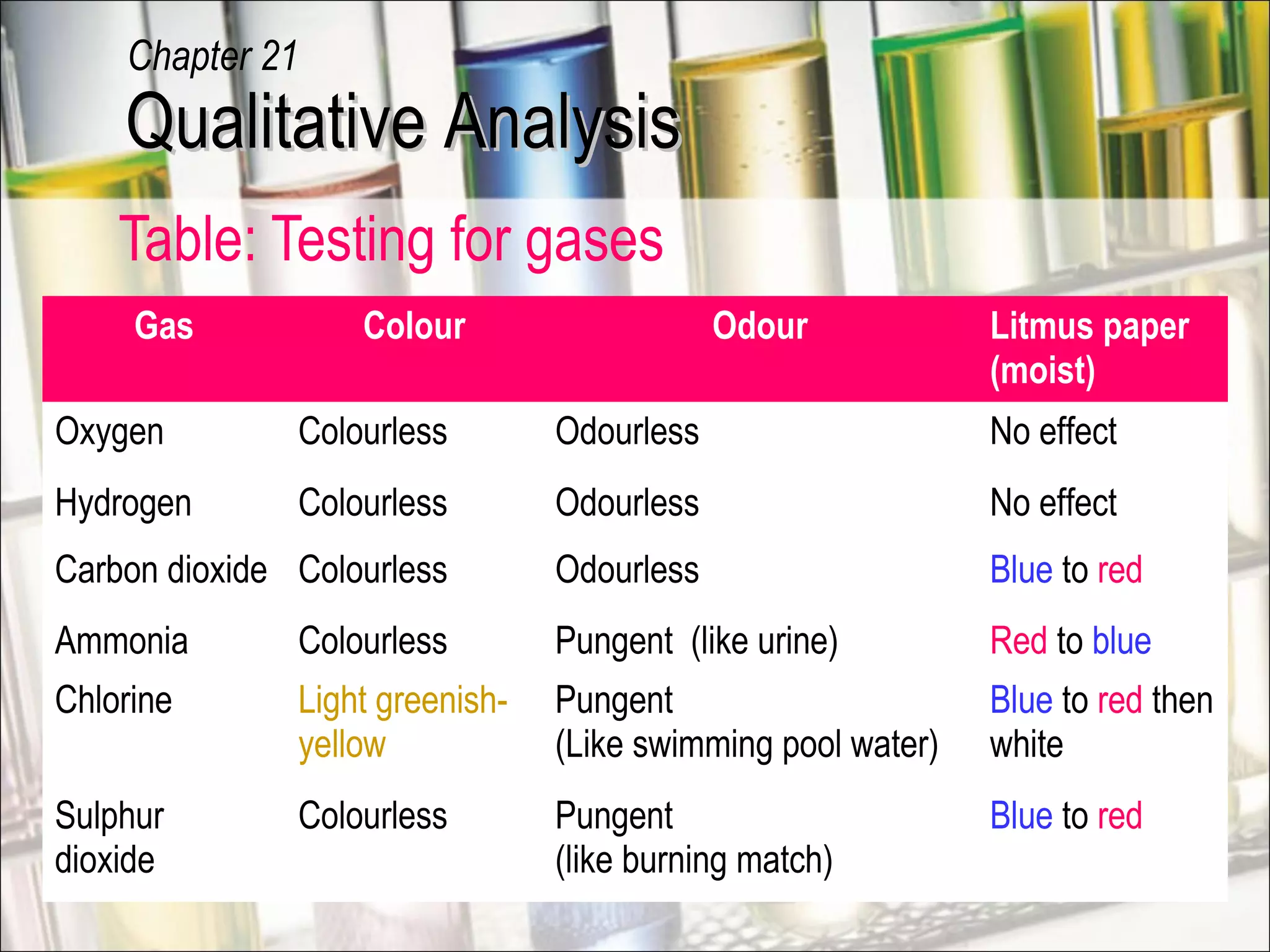
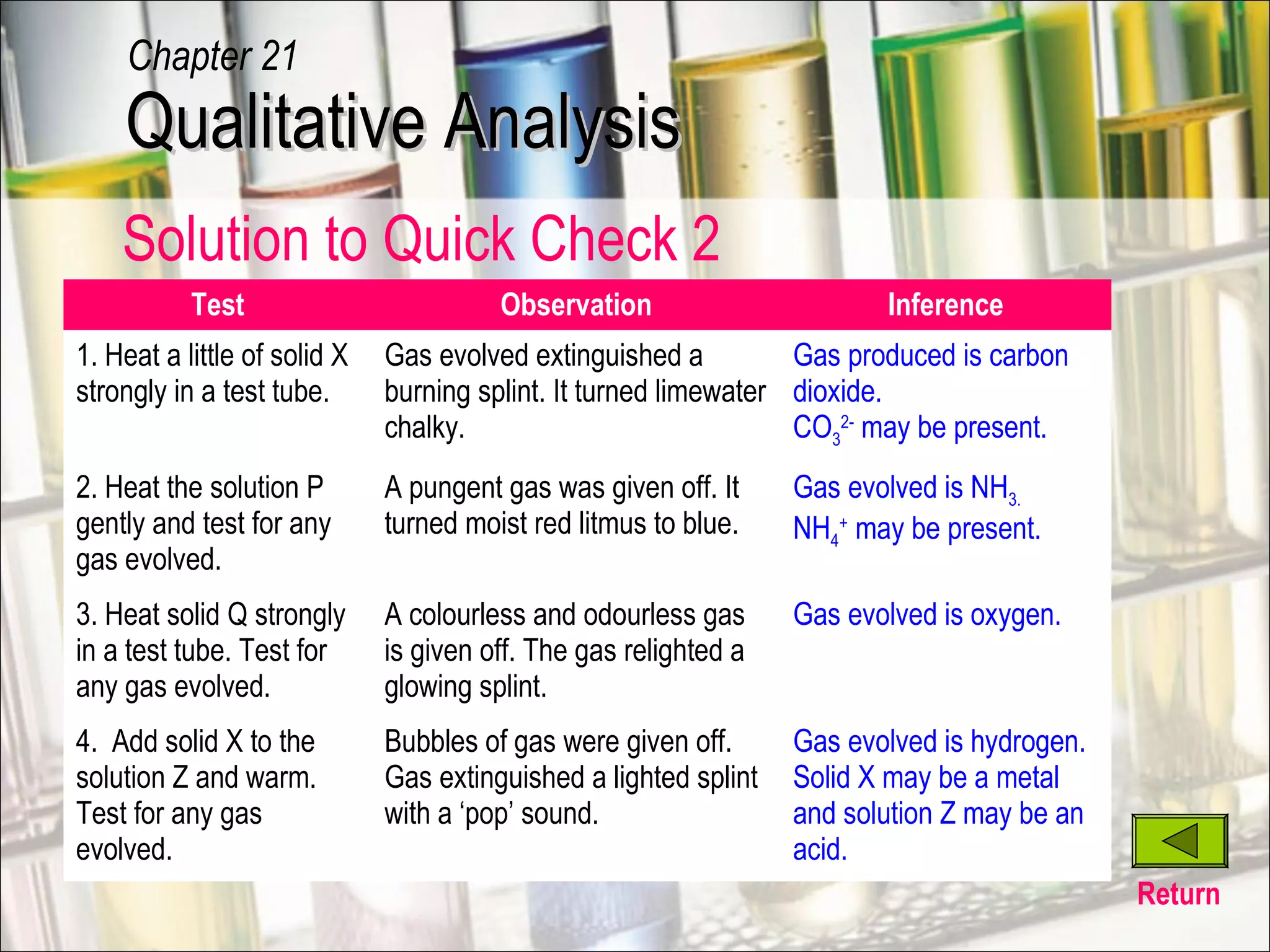
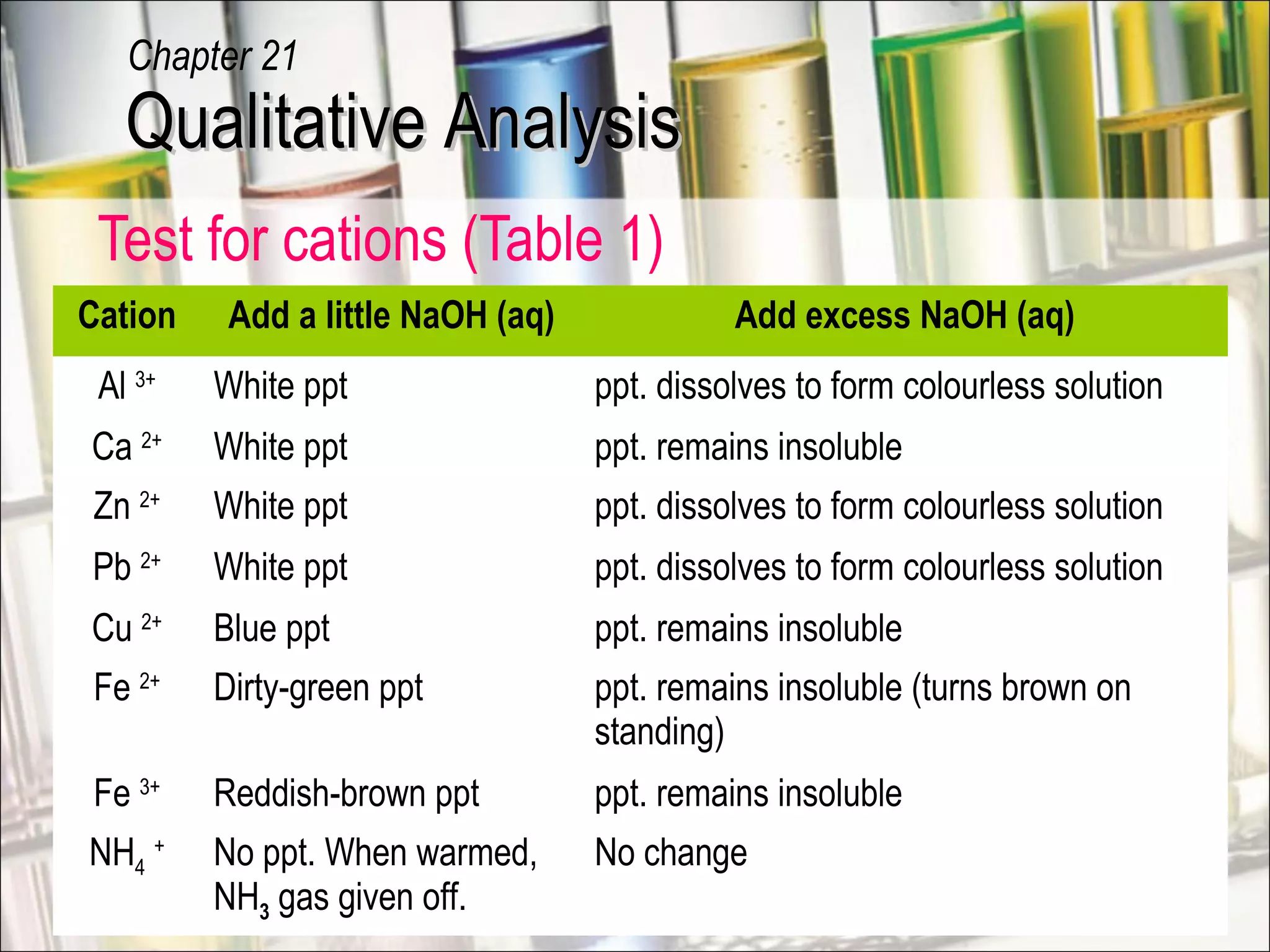
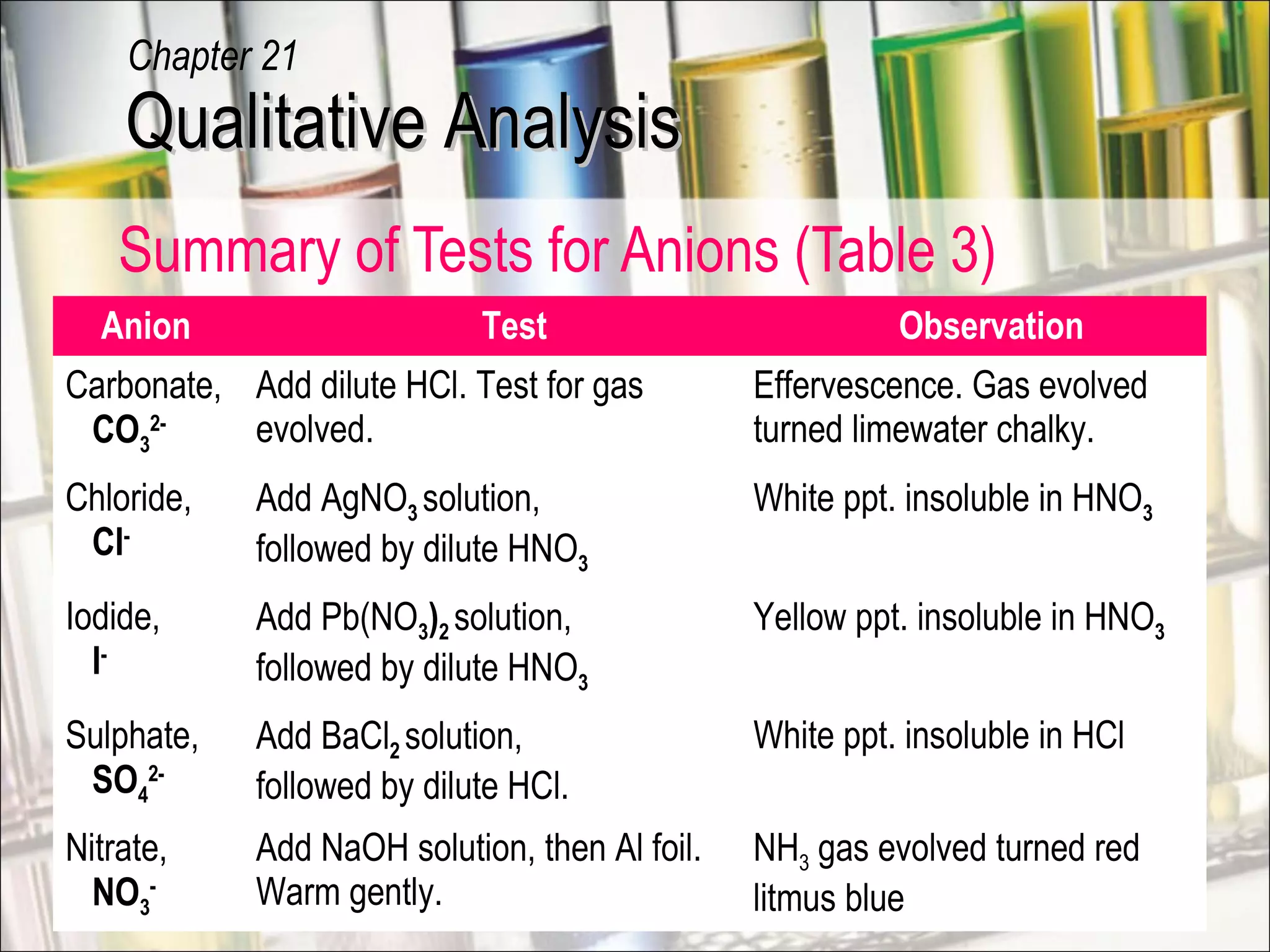
Qualitative analysis is a process used to identify unknown substances. It involves making preliminary observations of the unknown sample, carrying out a series of tests in a specific order, recording observations, and drawing conclusions to identify the cation(s) and anion(s) present. Key steps include testing for gases evolved, identifying cations using sodium hydroxide and ammonia solutions to produce precipitates of different colors and solubilities, and identifying anions using characteristic tests. Proper technique and accurate recording of observations are essential for correct identification of the unknown substance.