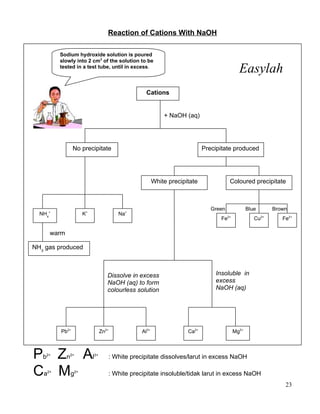
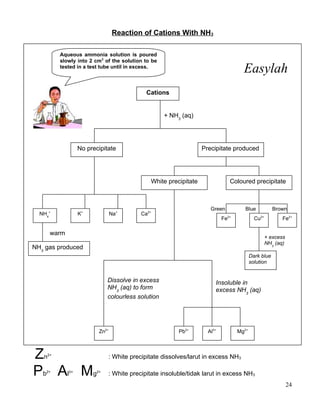
1. Salts can be either soluble or insoluble depending on their constituent ions. Soluble salts dissolve in water while insoluble salts do not.
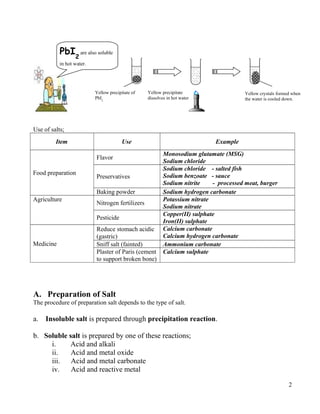
2. Insoluble salts are prepared through precipitation or double decomposition reactions between aqueous solutions containing the ions that will form the insoluble salt. Soluble salts are prepared through acid-base reactions or reactions between acids and reactive metals/metal oxides/carbonates.
3. Both soluble and insoluble salts require purification steps like filtration, rinsing, and drying to obtain the pure salt.









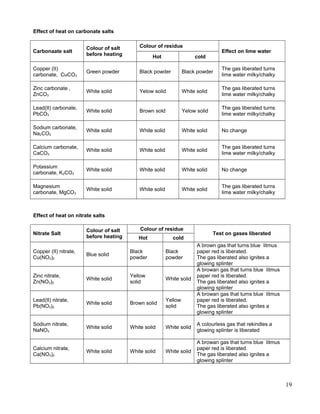






![3.9 g of potassium is burnt completely in the air as shown in the following equation;
4K(s) + O2(g) → 2K2O(s)
What is the mass of potassium oxide produced?
[Relative atomic mass: K, 39; O, 16]
Solutions
Tip: Solve the question step by step
Step 1: Write Chemical Equation
4K(s) + O2(g) → 2K2O(s)
4 mol of K react with 1 mol of O2 produce 2 mol K2O
Step 2: Calculate the number of mole
[Get the information from the question]
Step 3: Find the coefficient From Balance Chemical Equation
FBCE;
4 mol of K produce 2 mol K2O
Thus;
0.1 mol of K produce 2/4 mol K2O = 0.2 mol K2O
FBCE;
[Sebelah kiri] [Sebelah kanan]
Bil. mol yang telah dikira Bil. Mol yang hendak ditentukan
4 mol K = 2 mol K2O
0.1mol K = 2/4 x 0.1mol K2O = 0.05 mol K2O
No. of mol of K2O = 0.05 mol
Step 4: Solve the questions
Thus;
Mass of K2O = 0.05 mol × Molar mass
= 0.05 mol× 55 g mol-1
= 2.75 g
Example 3:
30
No. of mol K =
mass
Molar mass
=
3.9 g
39 gmol-1
0.1 mol=](https://image.slidesharecdn.com/notesupdatessalts-131006000433-phpapp01/85/Notes-updates-salts-30-320.jpg)
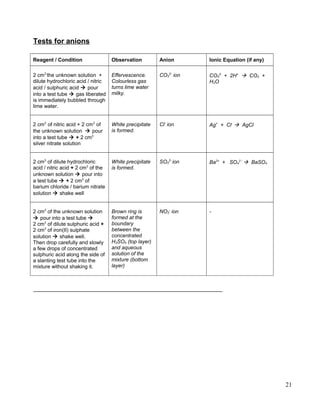
![Acids reacts with calcium carbonate, CaCO3 in limestone to form a salt and carbon dioxide, CO2.
A piece of limestone reacted completely with 100 cm3
of 31.5 g dm-3
nitric acid, HNO3.
[Relative atomic mass: H, 1; C, 12; N, 14; O, 16; Ca, 40. Molar volume: 24 dm3
mol-1
at room
conditions]
a. Calculate the mass of salt produced.
b. What is the volume of carbon dioxide, CO2 liberated at room conditions?
Step 1: Write Chemical Equation
Chemical Equation: 2HNO3 + CaCO3 → Ca(NO3)2 + CO2 + H2O
Step 2: Calculate the number of mole
Get the information from the question;
FBCE; 2HNO3 + CaCO3 → Ca(NO3)2 + CO2 + H2O
2 mol HNO3 = 1 mol Ca(NO3)2
0.05 mol HNO3 = ½ x 0.05 mol Ca(NO3)2 = 0.025 mol Ca(NO3)2
No. of mol of Ca(NO3)2 = 0.025 mol
Mass of Ca(NO3)2 = 0.025 mol × 40 + 2[14 + 3(16)] g mol-1
= 4.1 g
31
No. of mole of HNO3 =
Molarity × Volume
1000
=
0.5 mol dm-3
× 100 cm3
1000
= 0.05 mol
Concentration of HNO3 = 31.5 g dm3
=
Molar mass of HNO3
31.5 g dm3
= 0.5 mol dm-3
=
31.5 g dm3
1 + 14 + 48 g mol-1
Change the
concentration
given in g dm-3
to mol dm-3
first](https://image.slidesharecdn.com/notesupdatessalts-131006000433-phpapp01/85/Notes-updates-salts-31-320.jpg)
![FBCE; 2HNO3 + CaCO3 → Ca(NO3)2 + CO2 + H2O
2 mol HNO3 = 1 mol CO2
0.05 mol HNO3 = ½ x 0.05 mol CO2 = 0.025 mol CO2
No. of mol of CO2 = 0.025 mol
Volume of CO2 = 0.025 mol × 12 + 2(16) dm3
mol-1
= 1.1 dm3
Example 4:
Pb(NO3)2 compound decomposes when heated as shown in the following equation.
If 6.62 g of Pb(NO3)2 compound is heated, calculate;
[Relative atomic mass: N, 14; O, 16; Pb, 207; 1 mol of gas occupies 22.4 dm3
at s.t.p.]
(i) mass of PbO that is produced
(ii) volume of nitrogen dioxide produced at s.t.p
(ii) volume of oxygen produced at s.t.p
Solution:
FBCE; 2Pb(NO3)2 → 2PbO + 4NO2 + O2
2 mol Pb(NO3)2 = 2 mol PbO
0.02 mol Pb(NO3)2 = 0.02 mol PbO
No of mol PbO = 0.02 mol
Mass of PbO = 0.02 x 223 = 4.46 g
32
No of mol Pb(NO3
)2
=
mass
Molar mass
=
6.62 g
331 gmol-1
0.02 mol=
2Pb(NO3)2 → 2PbO + 4NO2 + O2](https://image.slidesharecdn.com/notesupdatessalts-131006000433-phpapp01/85/Notes-updates-salts-32-320.jpg)
![FBCE; 2Pb(NO3)2 → 2PbO + 4NO2 + O2
2 mol Pb(NO3)2 = 4 mol NO2
0.02 mol Pb(NO3)2 = 4/2 x 0.02 mol O2 = 0.04 mol O2
No of mol O2 = 0.04 mol
Volume of O2 = 0.04 x 22.4 dm3
= 0.896 dm3
// 896 cm3
FBCE; 2Pb(NO3)2 → 2PbO + 4NO2 + O2
2 mol Pb(NO3)2 = 1 mol O2
0.02 mol Pb(NO3)2 = ½ x 0.02 mol O2 = 0.01 mol O2
No of mol O2 = 0.01 mol
Volume of O2 = 0.01 x 22.4 dm3
= 0.224 dm3
// 224 cm3
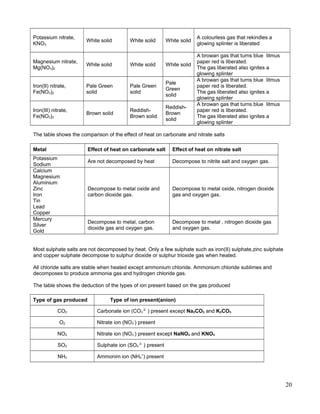
Numerical Problems involving stoichiometric reactions in the precipitation of salts
Question 1:
A student prepare copper(II) nitrate, Cu(NO3)2 by reacting copper(II) oxide, CuO with 200 cm3
of 2.0
moldm-3
nitric acid, HNO3. Calculate the mass of copper(II) oxide, CuO needed to react completely
with the acid.
[Relative atomic mass: Cu, 64 ; O, 16]
Question 2:
X cm3
of 0.5 moldm-3
sulphuric acid, H2SO4 is added to 100 cm3
of 1.0 moldm-3
lead(II) nitrate
solution to produce lead(II) sulphate, PbSO4.
[Relative atomic mass: Pb, 20; O, 16; S, 32]
a. Calculate the value of X.
b. Calculate the mass of lead(II) sulphate obtained.
Start to do exercises from any book.
I will help and guide you to master this topic.
Prepared by;
Kamal Ariffin Bin Saaim
SMKDBL
33](https://image.slidesharecdn.com/notesupdatessalts-131006000433-phpapp01/85/Notes-updates-salts-33-320.jpg)