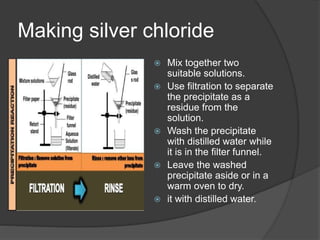
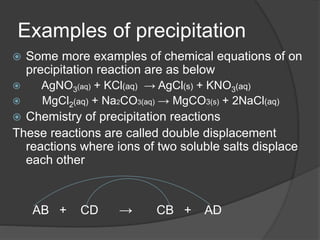
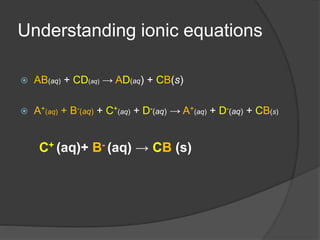
The document outlines two methods for preparing soluble salts and key concepts related to precipitation reactions. It describes how to make an insoluble salt through the reaction of two soluble salts and highlights which salts are soluble or insoluble. Additionally, it discusses the process of filtration and provides examples of precipitation reactions and their chemical equations.