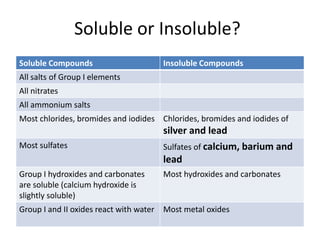
The document discusses four methods for preparing salts: 1) Reacting a metal with an acid, 2) Reacting an insoluble base with an acid, 3) Neutralizing an alkali with an acid through titration, and 4) Precipitation. It then provides examples of soluble and insoluble compounds, and explains how to specifically prepare zinc sulfate by reacting zinc powder with sulfuric acid. The document asks to describe how to prepare several example salts using these methods.