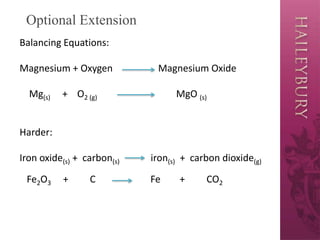
The document discusses word equations used in year 8 science to represent chemical reactions, detailing the structure and components, such as reactants and products. It emphasizes the importance of indicating the state of matter with subscripts and provides examples of converting sentences into word equations. Additionally, it encourages classroom practice and offers an optional extension on balancing equations.