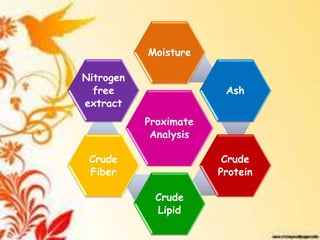
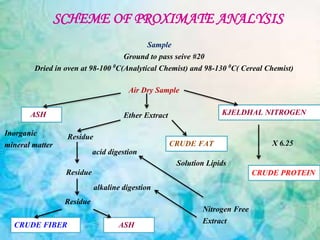
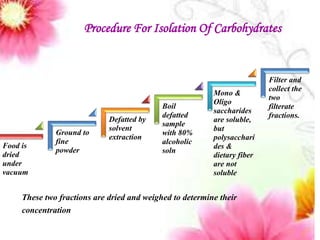
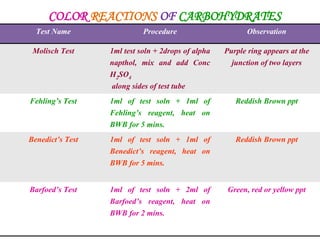
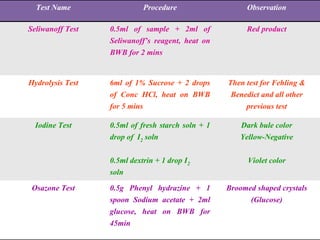
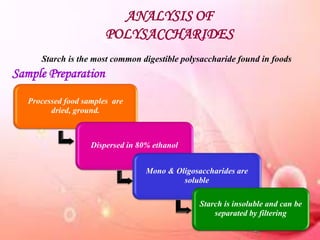
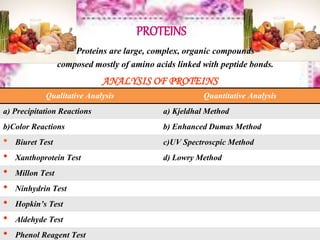
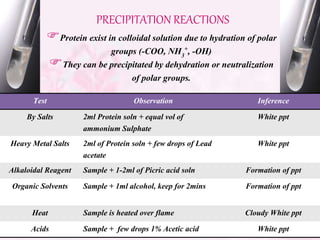
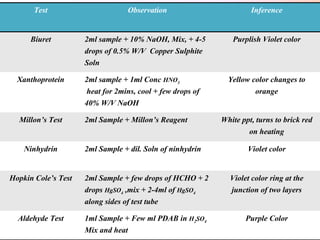
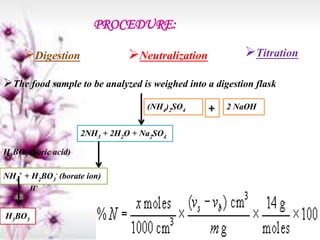
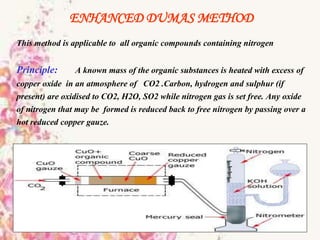
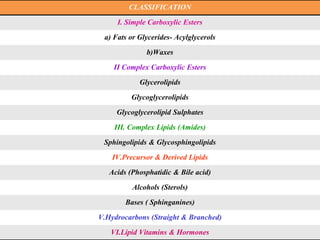
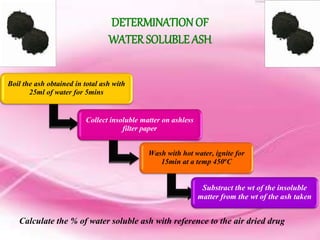
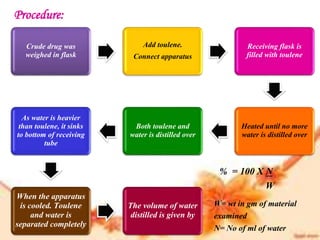
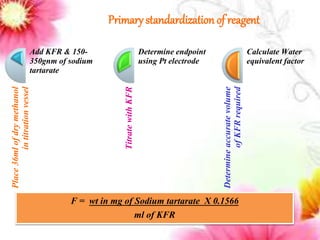
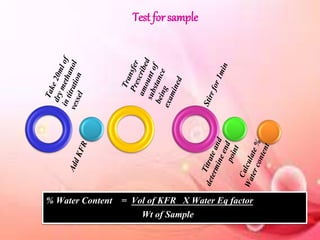
This document provides an overview of proximate analysis to determine macronutrients like carbohydrates, proteins, fats, and moisture content. It discusses the classification, isolation, and various quantitative analysis methods for each macronutrient. Proximate or Weende analysis partitions food compounds into moisture, ash, crude protein, crude lipid, crude fiber, and nitrogen-free extract. Common techniques described include Kjeldahl method for protein, Soxhlet extraction for fat, gravimetric methods for carbohydrates, and determining ash content.