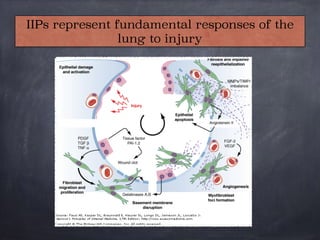
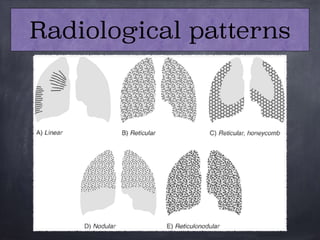
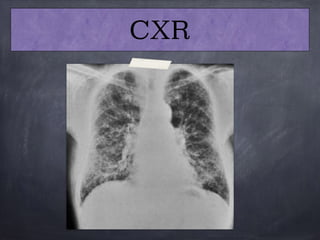
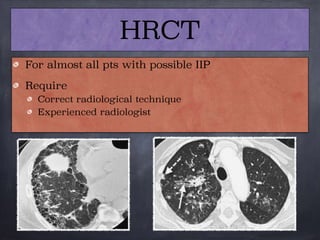
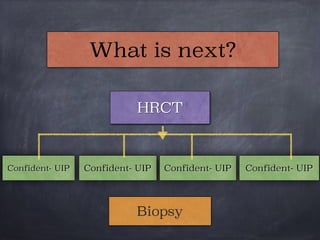
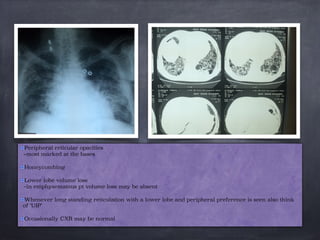
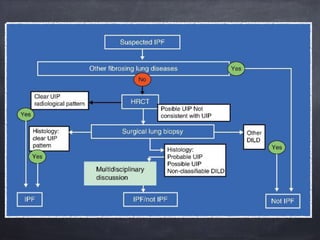
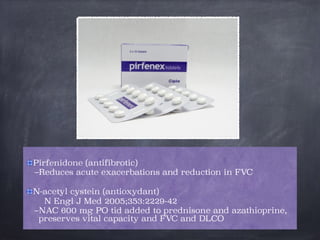
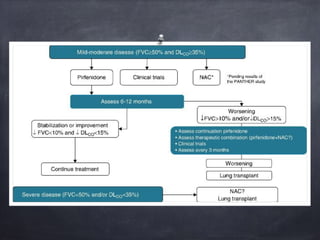
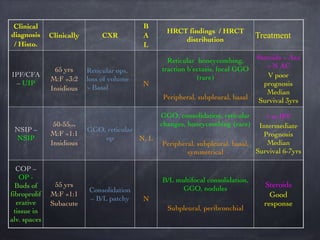
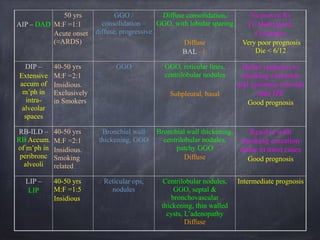
This document discusses interstitial lung diseases (ILD), particularly focusing on idiopathic interstitial pneumonias (IIPs), their classifications, diagnostic processes, and management strategies. It outlines the characteristics of various diseases, including idiopathic pulmonary fibrosis (IPF), non-specific interstitial pneumonia (NSIP), and acute interstitial pneumonia (AIP), highlighting the importance of histological and radiological examinations in diagnosis. Moreover, it reviews treatment options such as antifibrotic therapies and emphasizes the need for careful diagnosis to prevent unnecessary treatments.