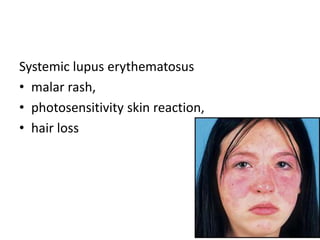
Interstitial lung disease (ILD) refers to over 100 lung disorders that share clinical, radiographic, and pathological features. ILDs can be classified based on histopathology, clinical characteristics, or the ATS/ERS classification system. Presentation involves nonspecific symptoms like dyspnea and cough. Diagnosis involves clinical history, imaging like HRCT, pulmonary function tests, bronchoscopy, and sometimes surgical lung biopsy. Treatment depends on underlying cause but may include immunosuppression, antifibrotic drugs, managing comorbidities, and lung transplantation for advanced cases.