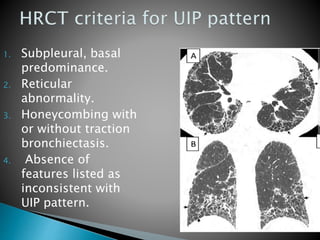
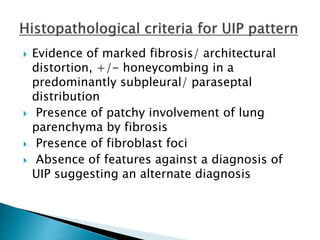
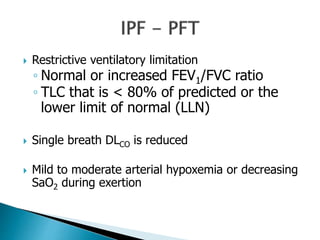
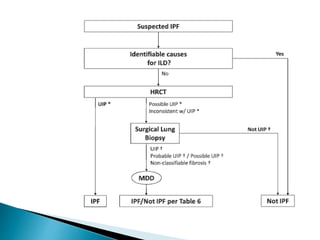
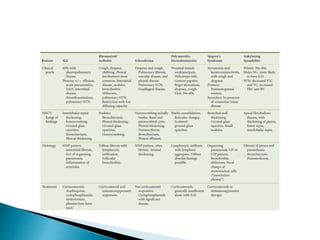
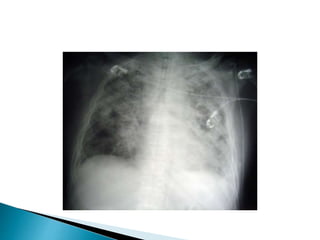
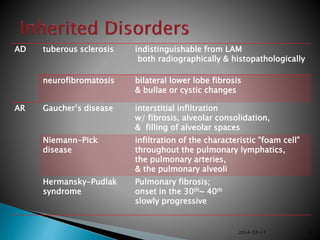
1. Interstitial lung diseases (ILDs) involve the lung parenchyma including the alveoli, capillaries, and tissues between them.
2. Patients commonly present with progressive dyspnea, cough, and interstitial opacities on imaging.
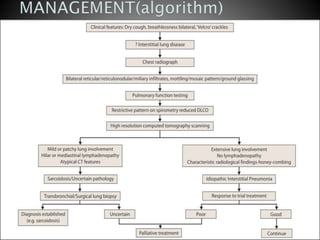
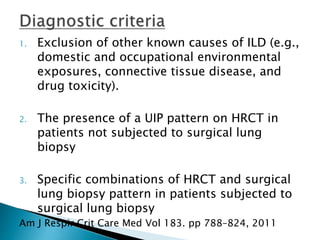
3. A thorough evaluation includes pulmonary function tests, imaging, biopsy, and ruling out other known causes to identify the underlying ILD.