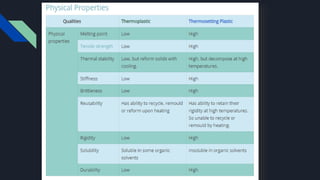
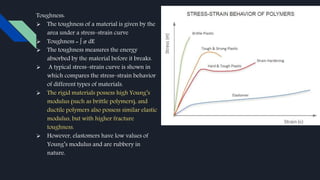
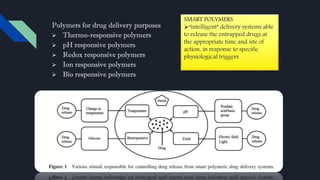
Polymers have played an integral role in advancing drug delivery technology by providing remote control of drug release. Polymers can conjugate to therapeutics to improve their pharmacokinetic and pharmacodynamic properties through increased plasma half-life, protection from enzymes, reduced immunogenicity, and potential for targeted delivery. Polymers are composed of repeating monomer units connected by covalent bonds and can be classified based on their monomer composition, method of polymerization, architecture, application, morphology, and degradability. Common polymers used in drug delivery systems include PEG, PLGA, chitosan, and HPMC.
















![ Diene elastomers are polymerized from monomers containing two sequential double
bonds.
Eg: polyisoprene, polybutadiene, and polychloroprene.
Non-diene elastomers have no double bonds in the structure, and thus, crosslinking
requires other methods than vulcanization such as addition of trifunctional monomers
(condensation polymers), or addition of divinyl monomers (free radical polymerization), or
copolymerization with small amounts of diene monomers like butadiene.
Eg: butyl rubber (polyisobutylene), polysiloxanes (silicone rubber), polyurethane
(spandex), and fluoro-elastomers.
Thermoplastic elastomers (TPE) are a class of copolymers or a physical mix of
polymers (usually a plastic and a rubber) that consist of materials with
both thermoplastic and elastomeric properties. They contain rigid (hard) and soft (rubbery)
repeat units
Eg: SIS(Styrene-Isoprene-Styrene) and SBS [Poly(styrene-butadiene-styrene)]](https://image.slidesharecdn.com/polymers-211117133253/85/Polymers-19-320.jpg)






















![pH-Responsive basic polymers
● Weak polybases, which undergo ionization/deionization transitions from pH ∼7–11, are
utilized as pH-responsive polymers.
● The amine groups are located in their side chains and these groups accept protons at low
pH values by forming poly-electrolytes releasing them under basic conditions.
● Eg: poly[(2-dimethylamino)ethyl methacrylate] PDMA, poly[(2-diethylamino)ethyl
methacrylate] (PDEA), and poly[(2-diisopropylamino)ethyl methacrylate] (PDPA)
pH-Responsive natural polymers
● On suitable chemical modification, these polymers can provide better materials for drug
delivery systems.
● Eg: Alginic acid, Chitosan, CMC, HA](https://image.slidesharecdn.com/polymers-211117133253/85/Polymers-44-320.jpg)






















