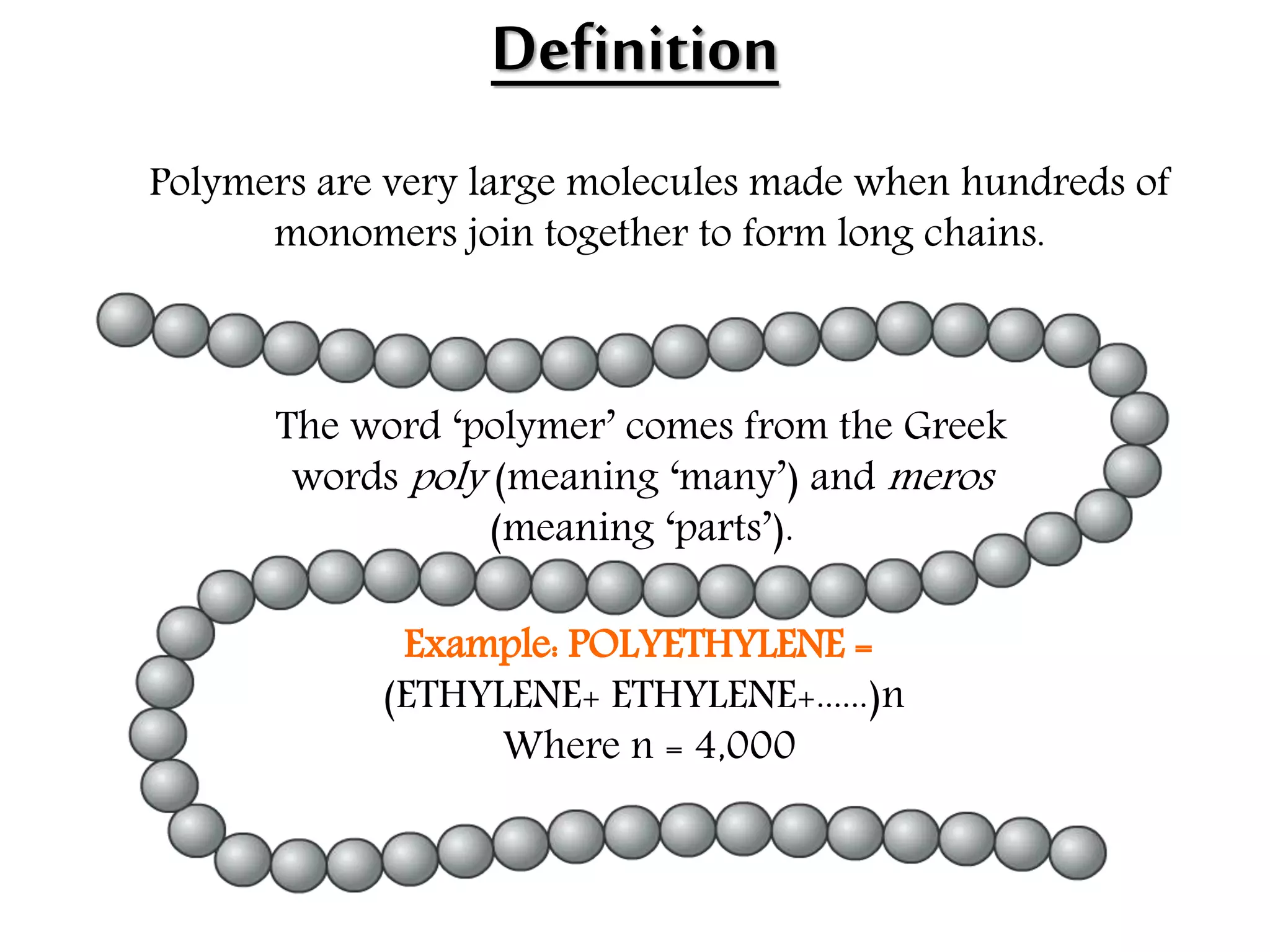
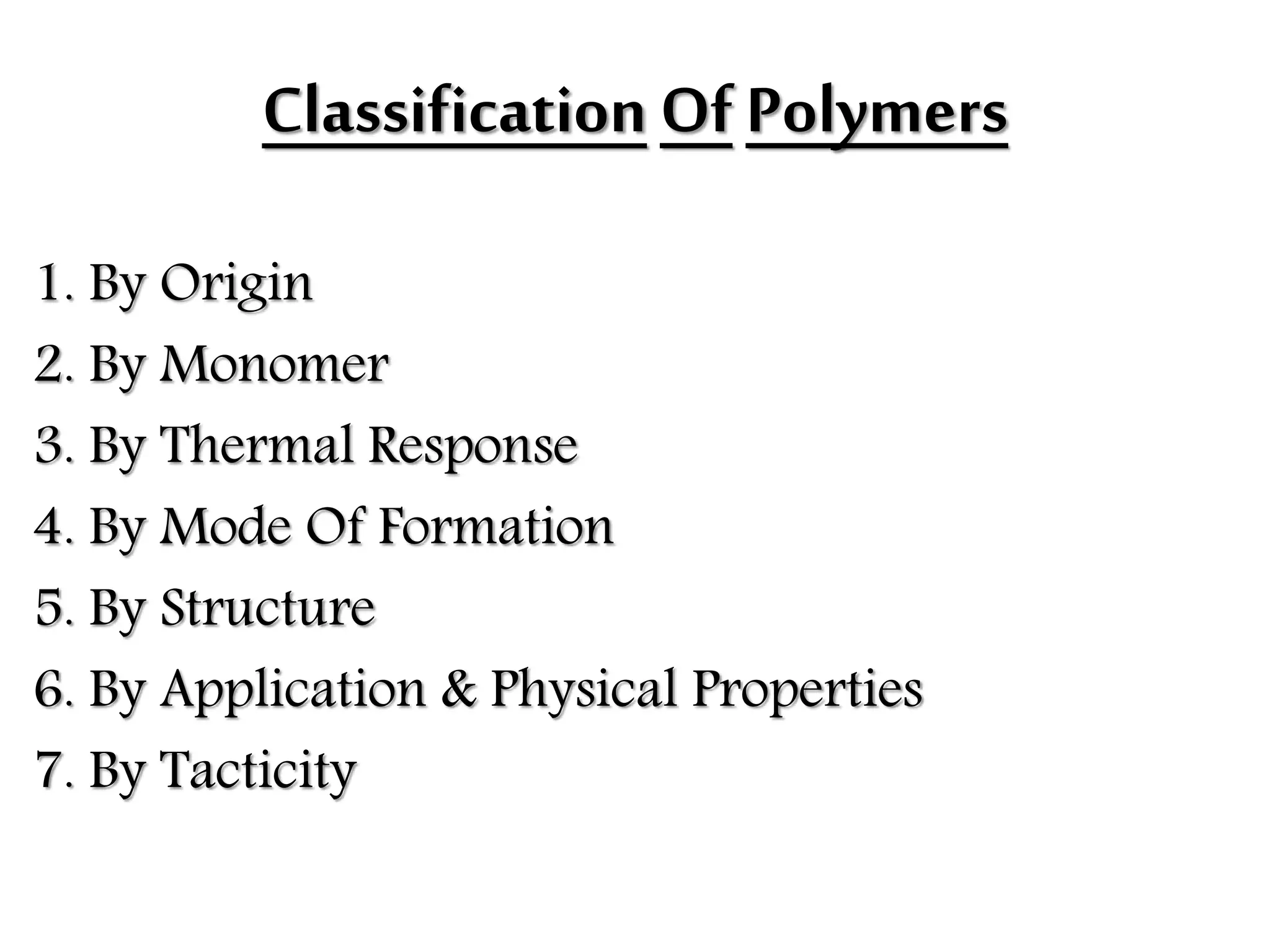
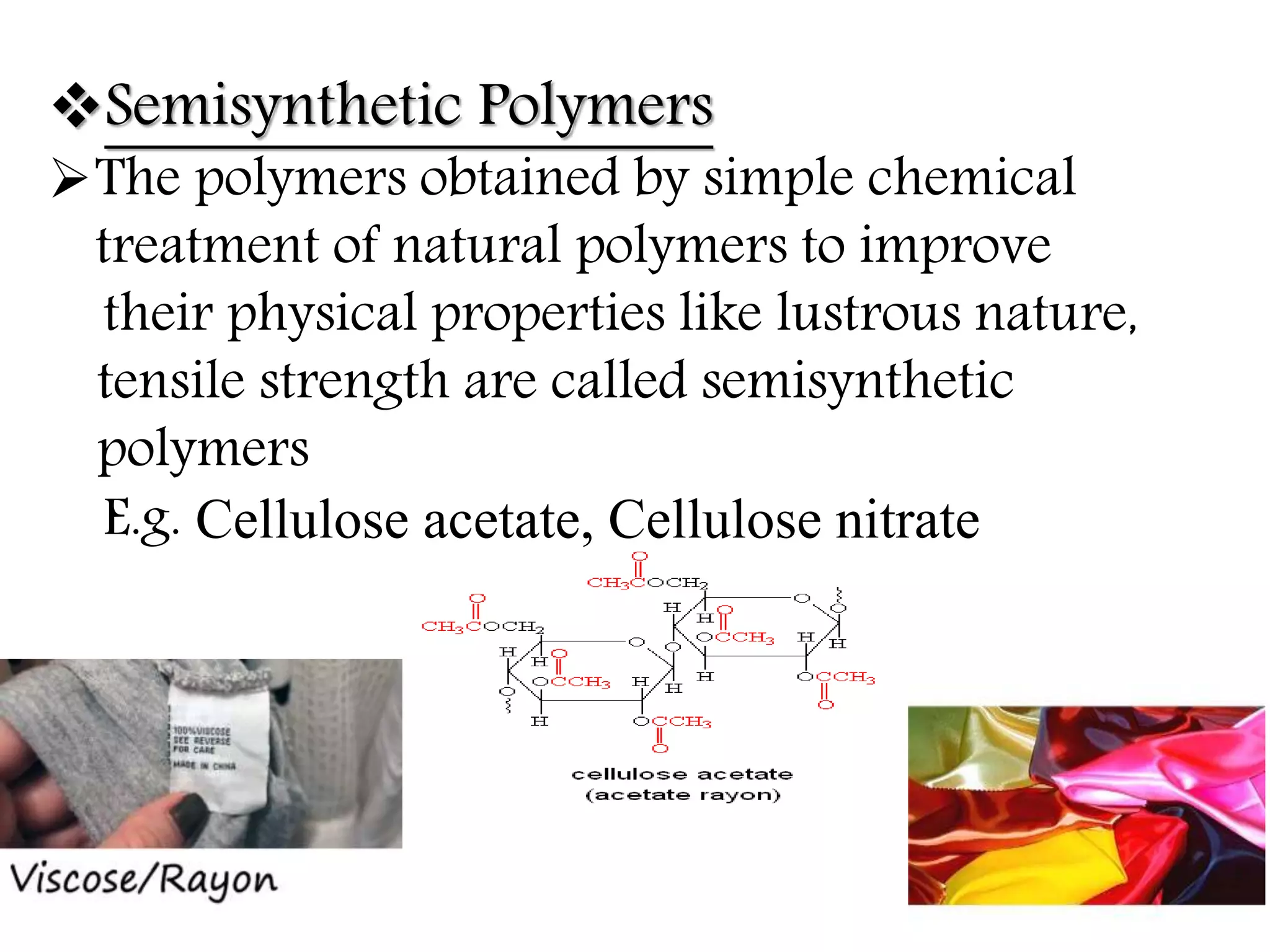
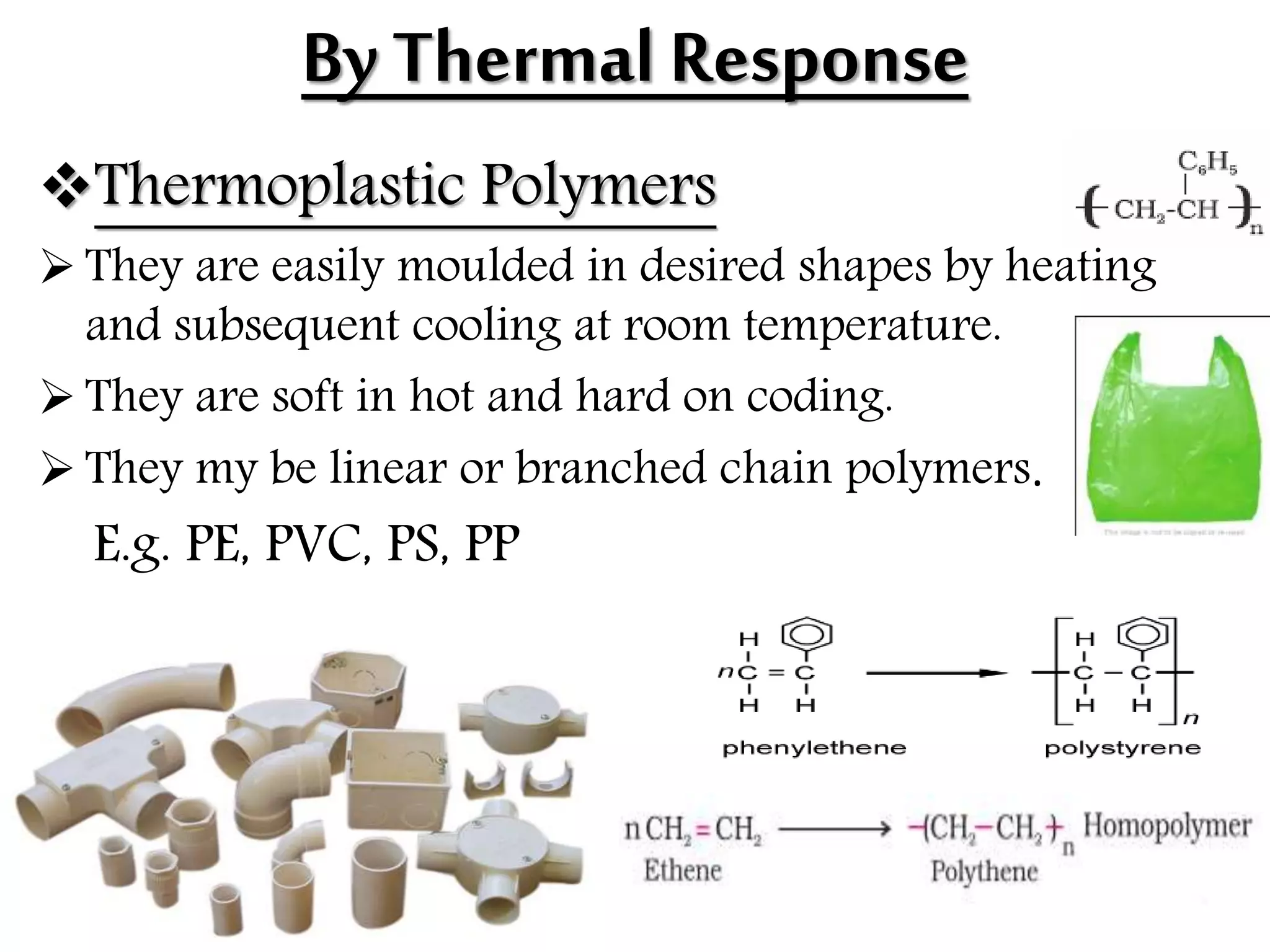
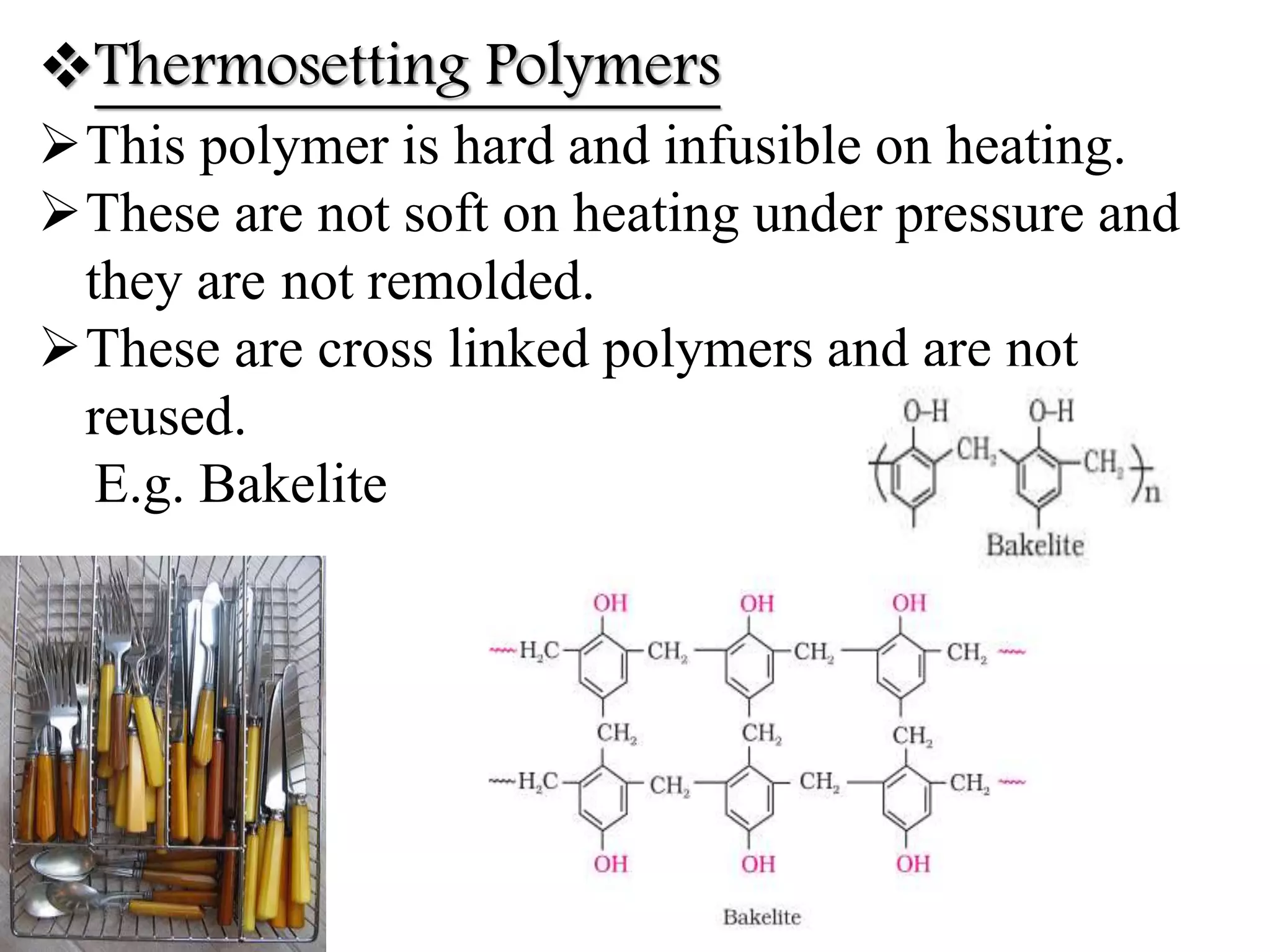
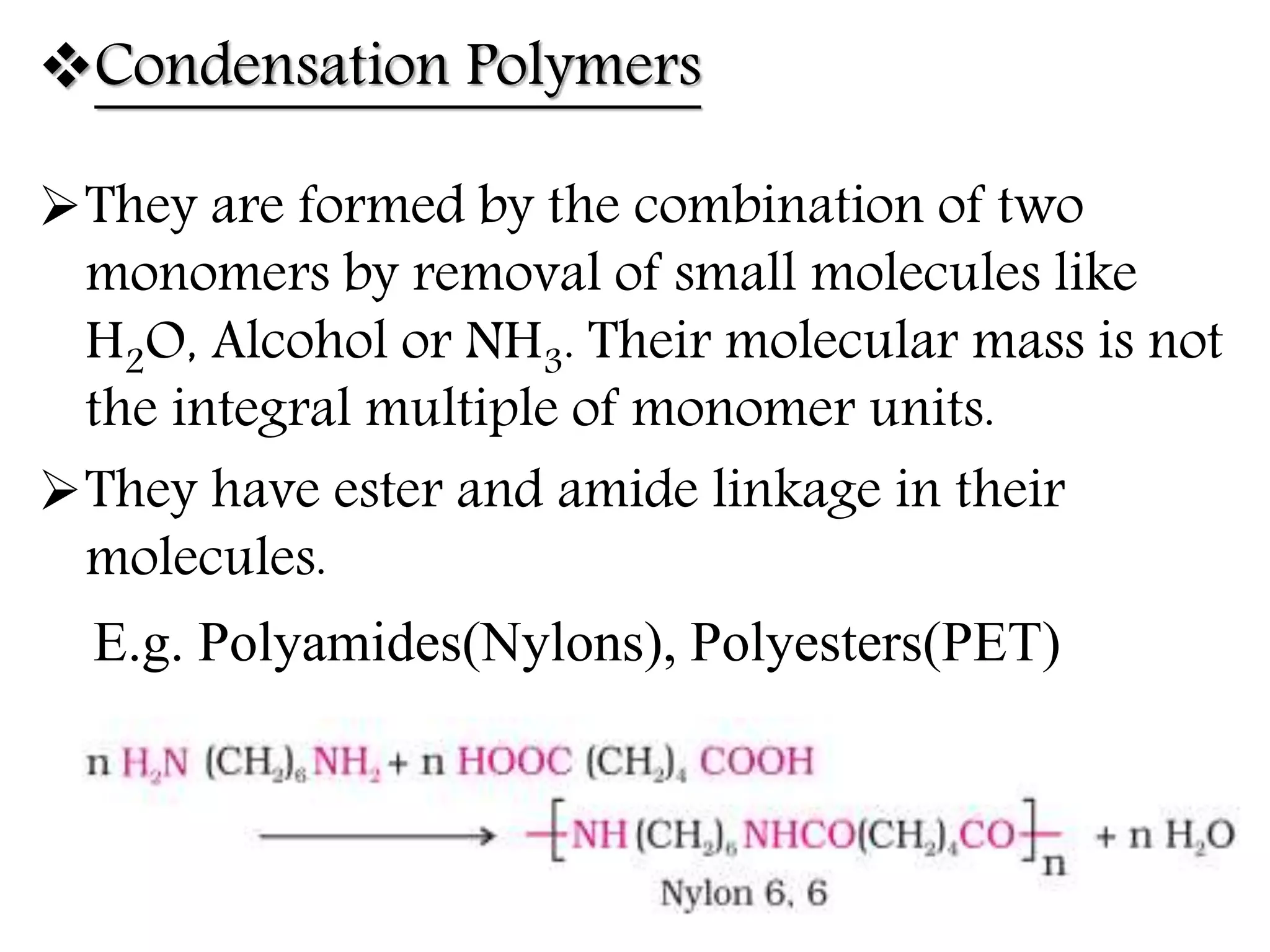
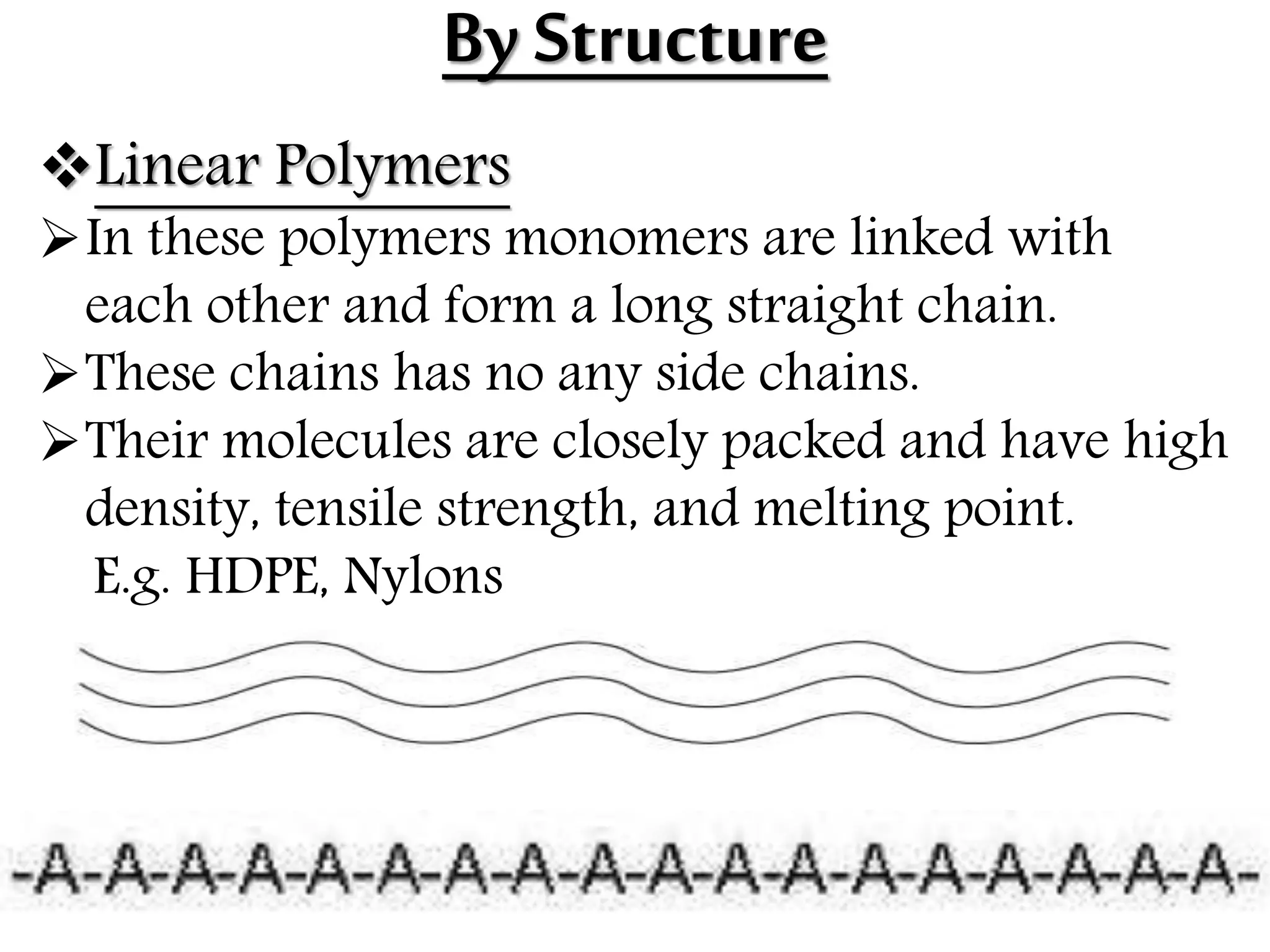
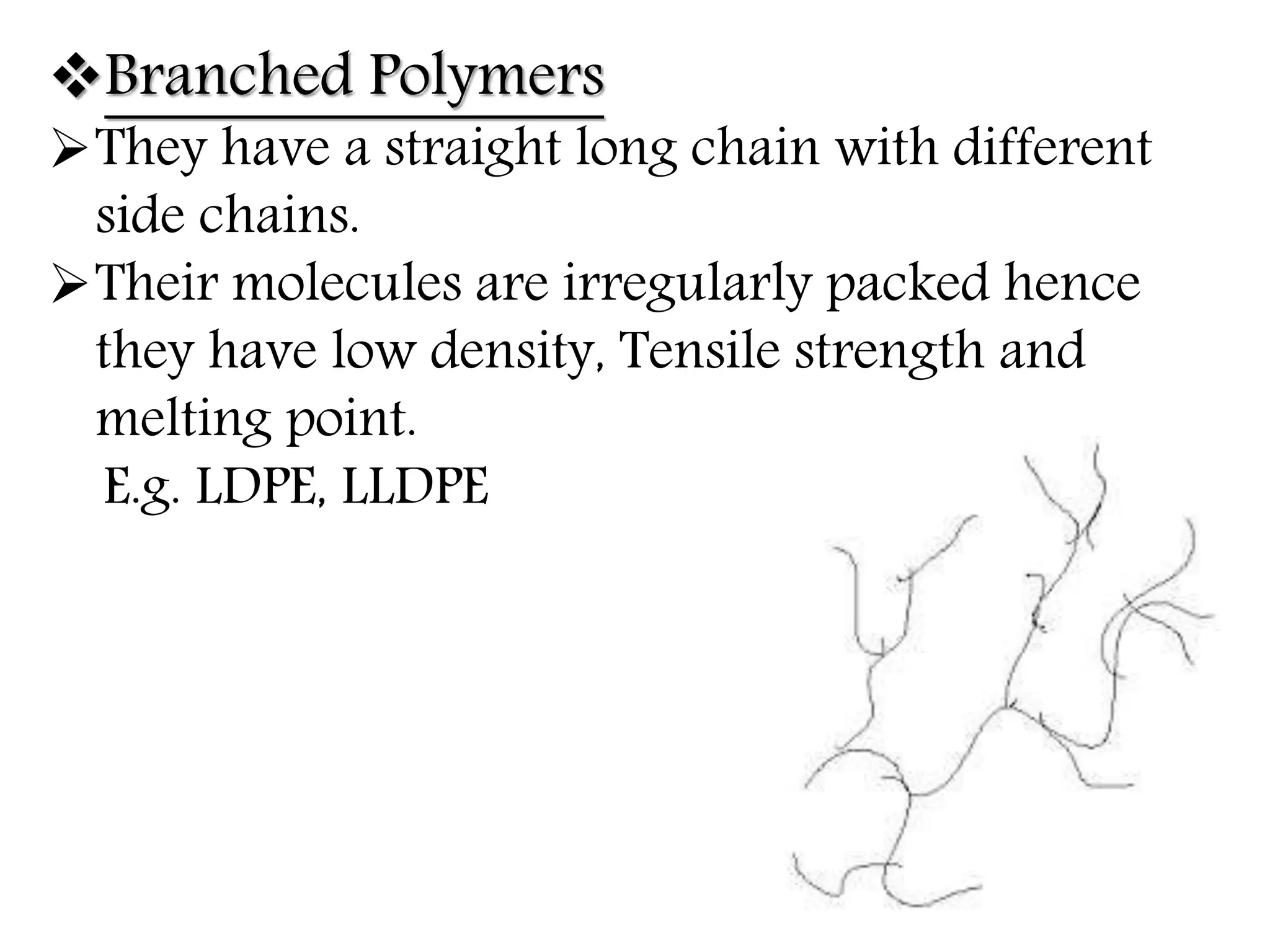
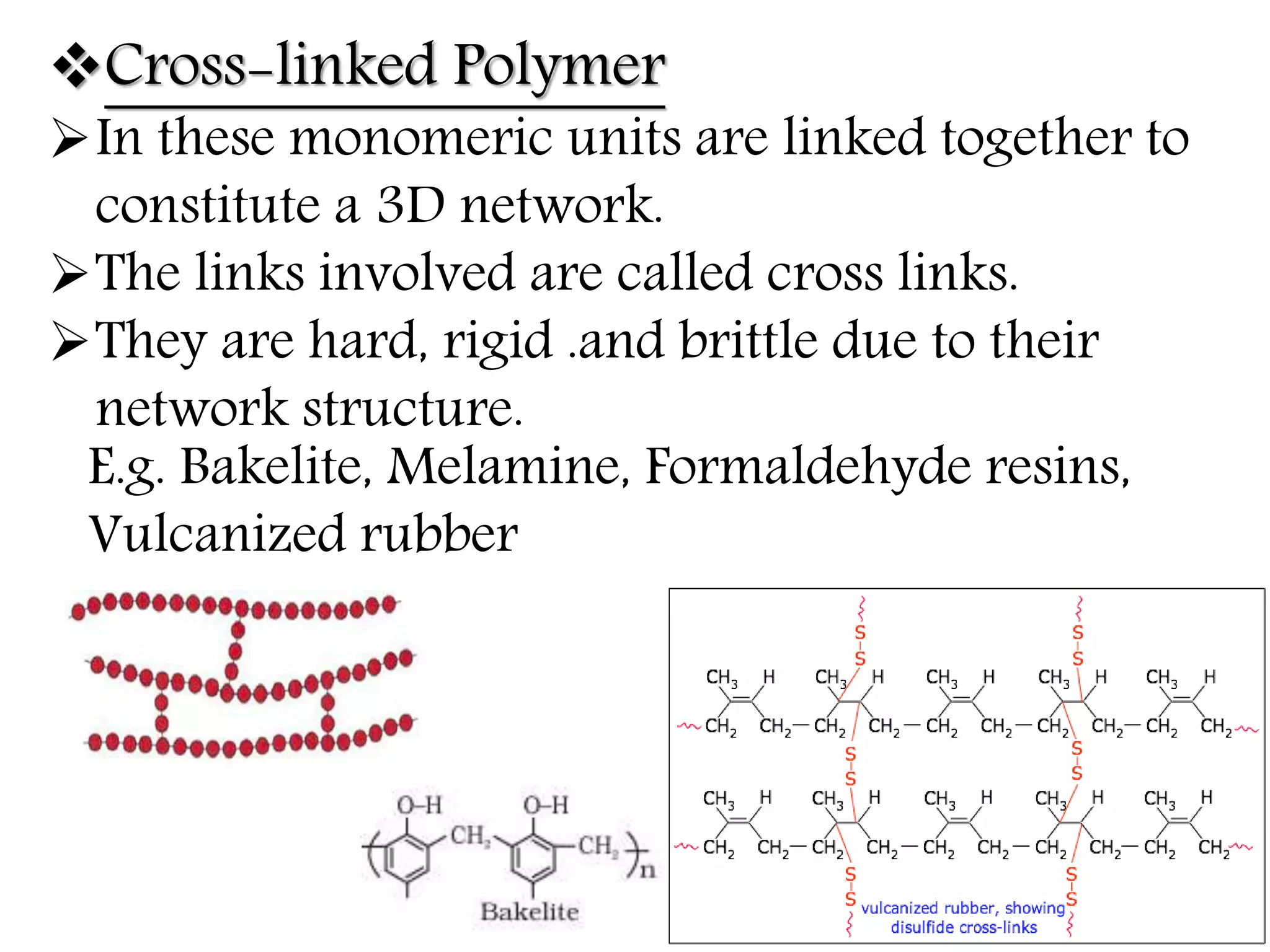
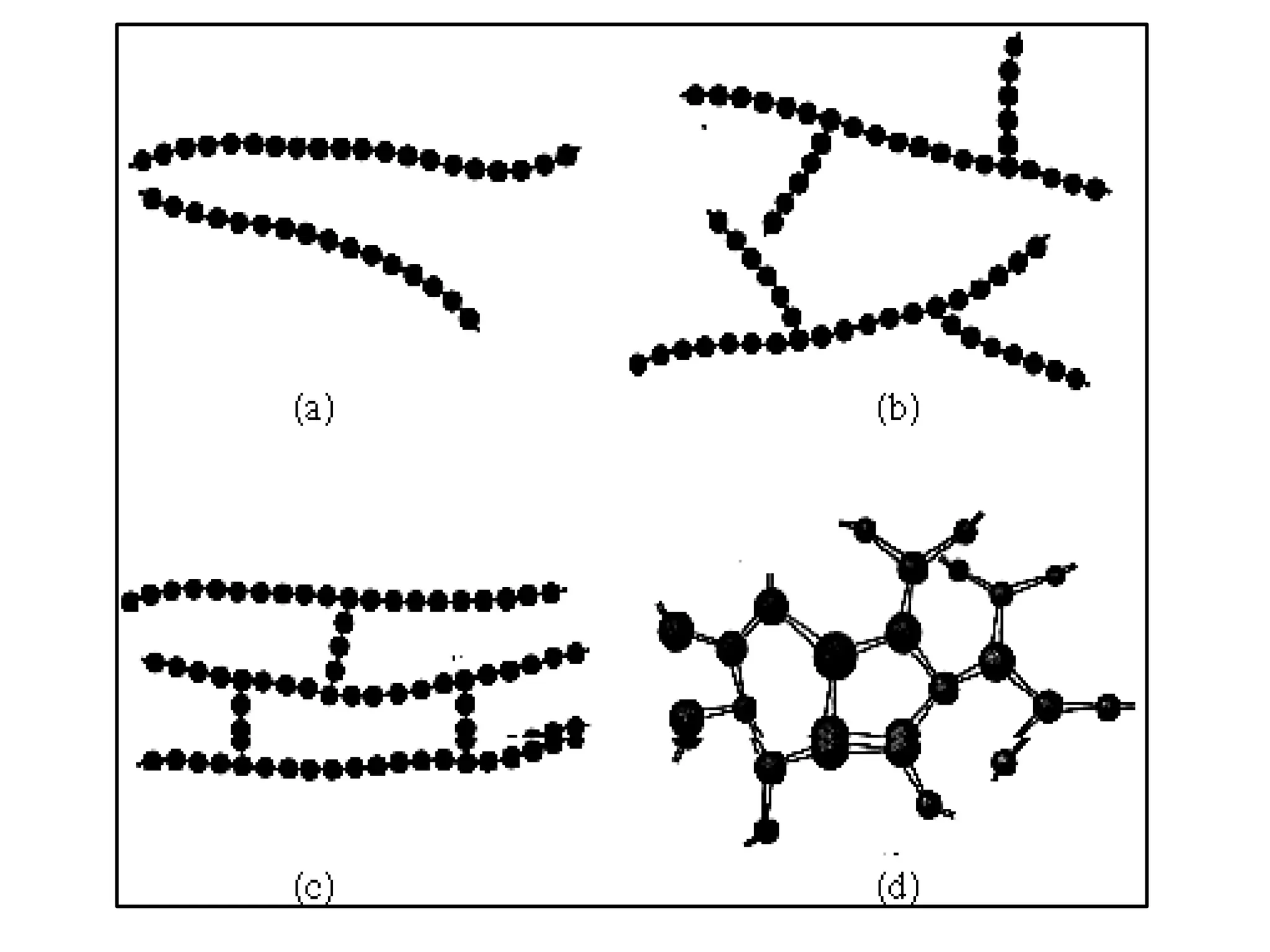
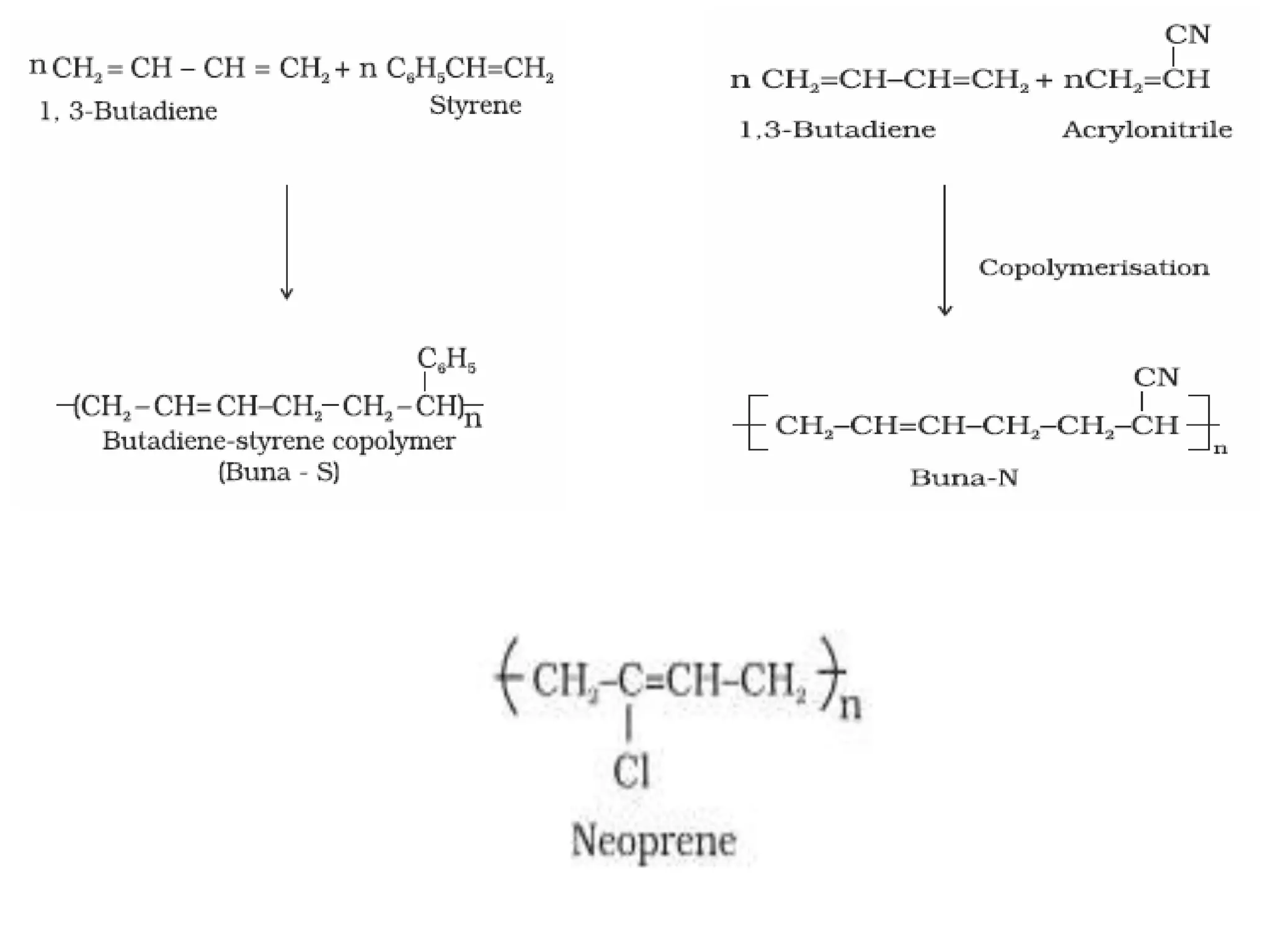
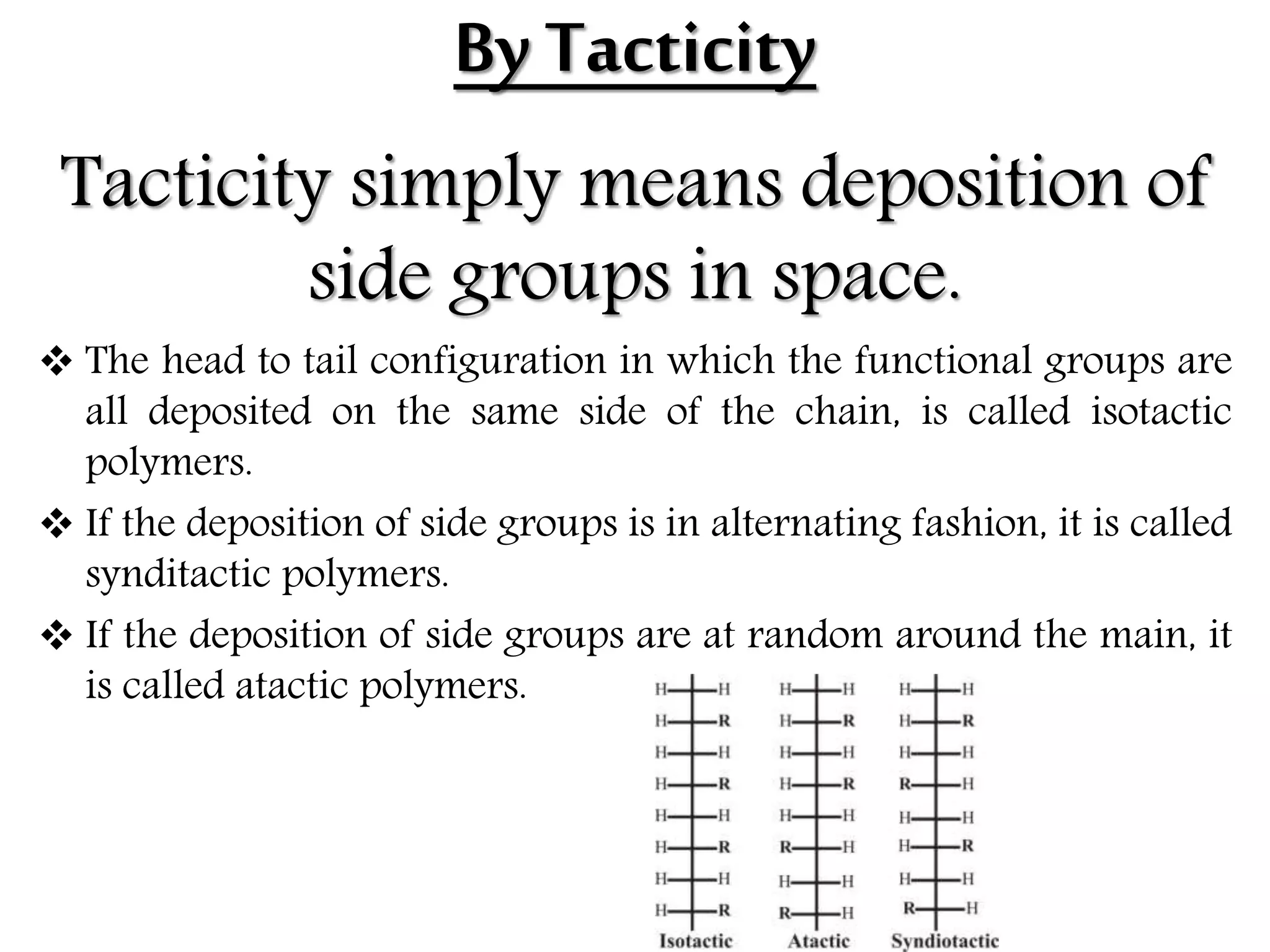
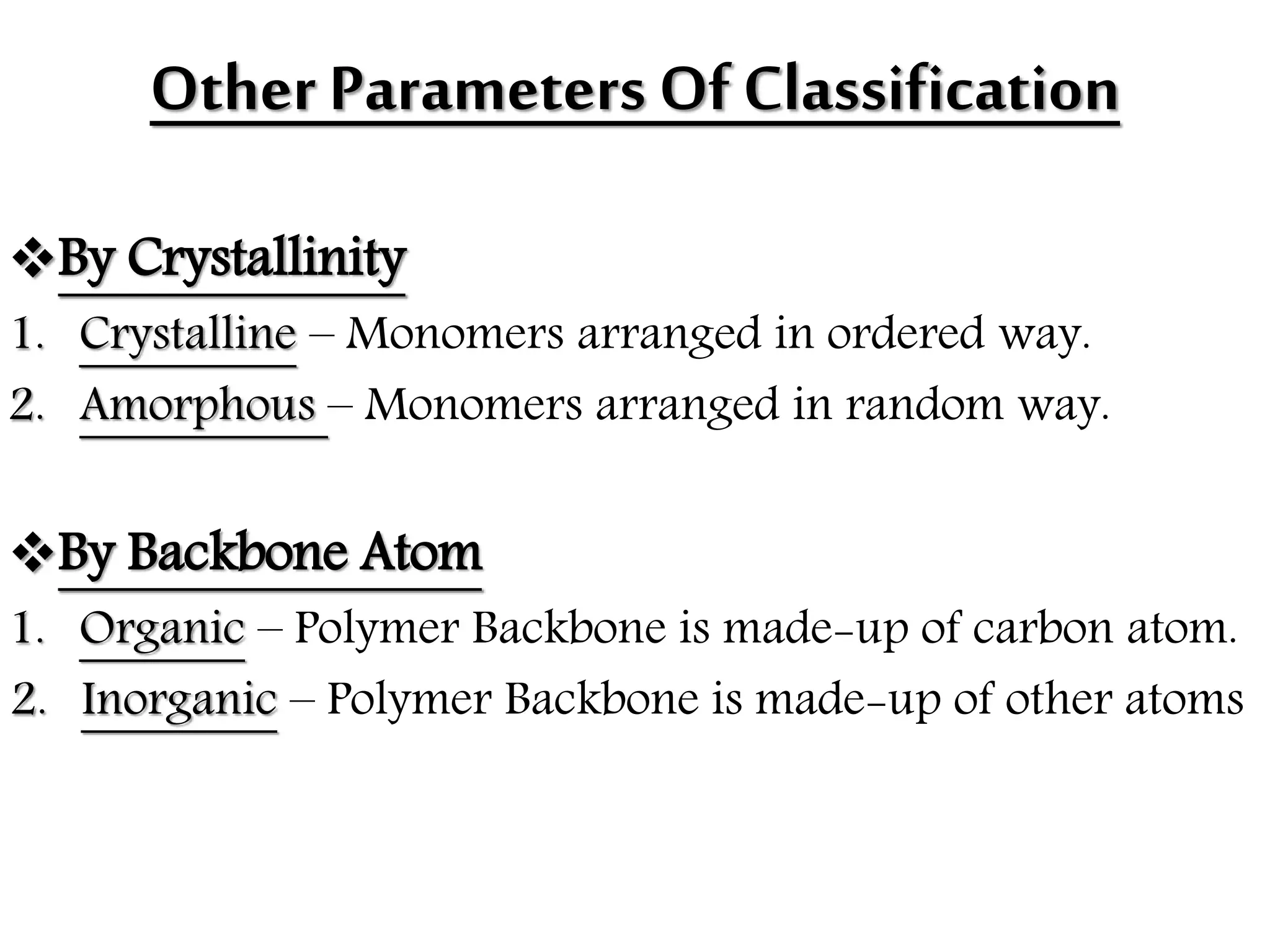
The document by Devansh Gupta presents a detailed classification of polymers, defining key terms such as polymers, monomers, and polymerization, alongside various classification criteria including origin, monomer type, thermal response, mode of formation, structure, application, physical properties, and tacticity. Natural, semisynthetic, and synthetic polymers are discussed, as well as different types such as homopolymers and copolymers. The document also emphasizes the importance of polymers in applications like fibers, plastics, elastomers, and resins, along with references for further reading.