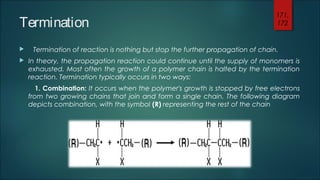
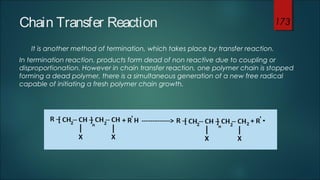
This document summarizes key aspects of polymer science including polymerization, monomers, and polymerization mechanisms. It discusses that polymerization is the process that links monomer molecules into polymer chains. There are different polymerization mechanisms including chain-growth and free radical polymerization. Chain-growth polymerization proceeds through initiation, propagation, and termination steps. Free radical polymerization uses initiators to generate free radicals to start the polymerization reaction. The document provides examples of monomers and initiators and discusses how functionality of monomers affects the structure of the resulting polymer chains.