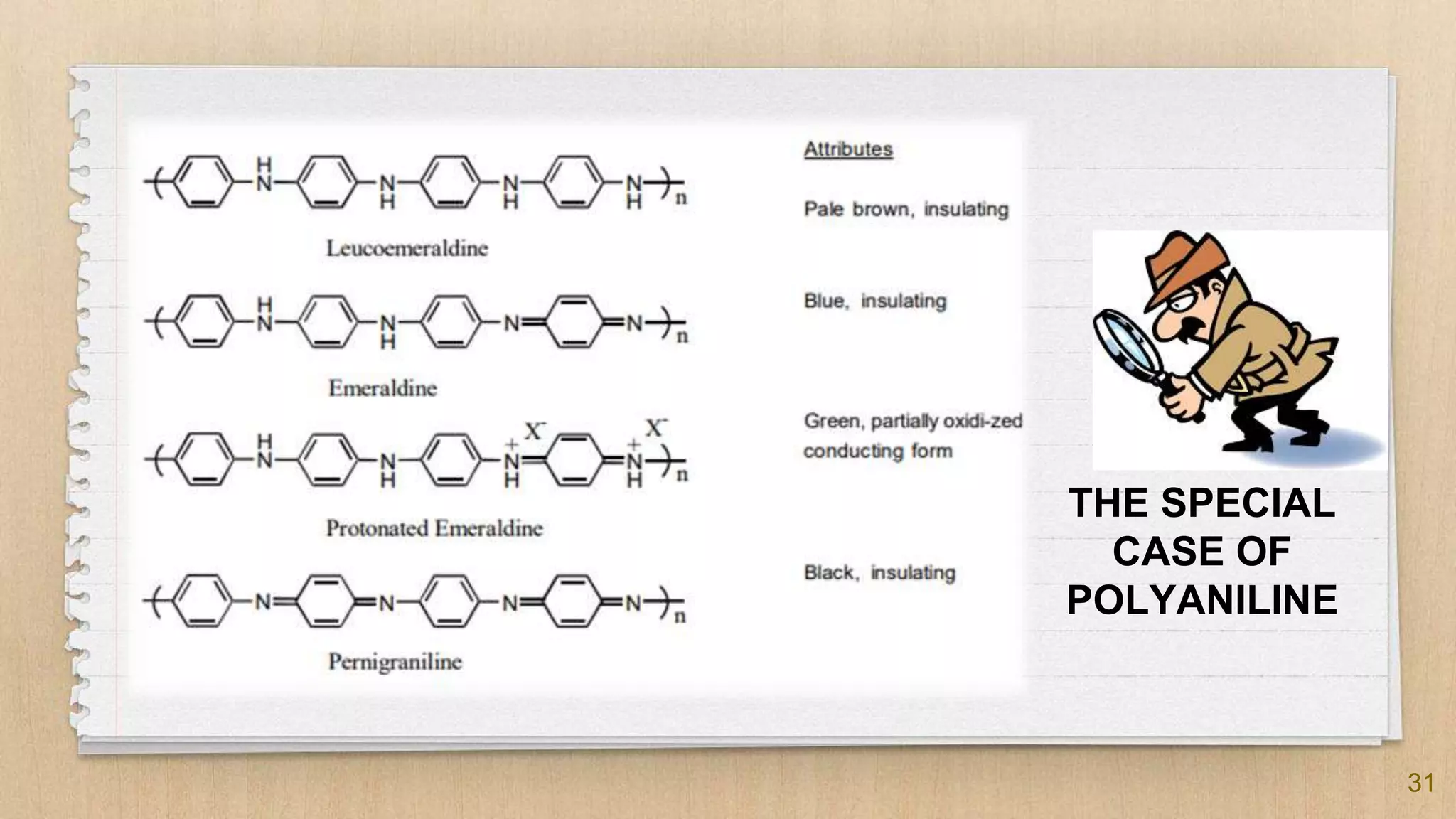
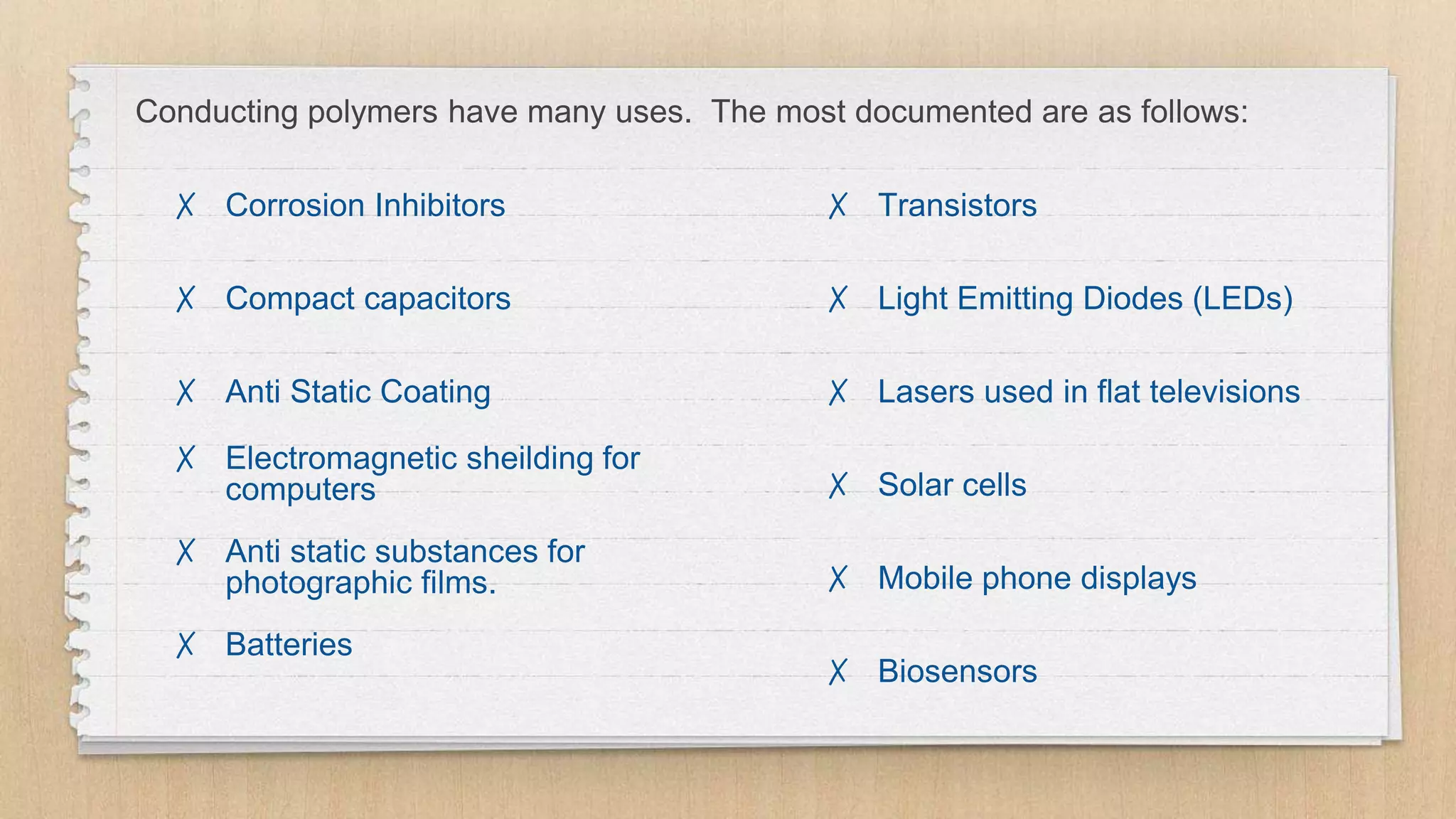
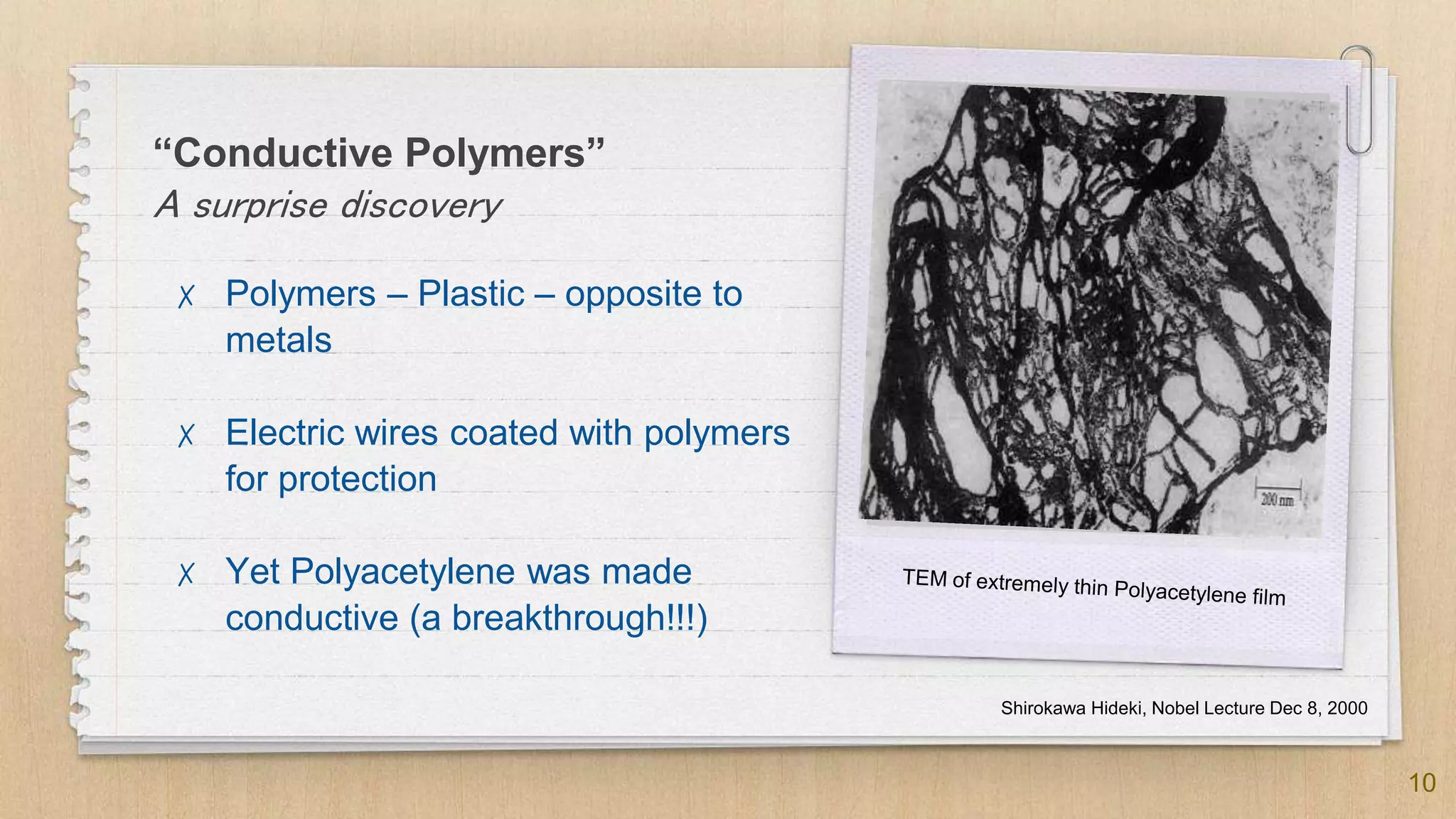
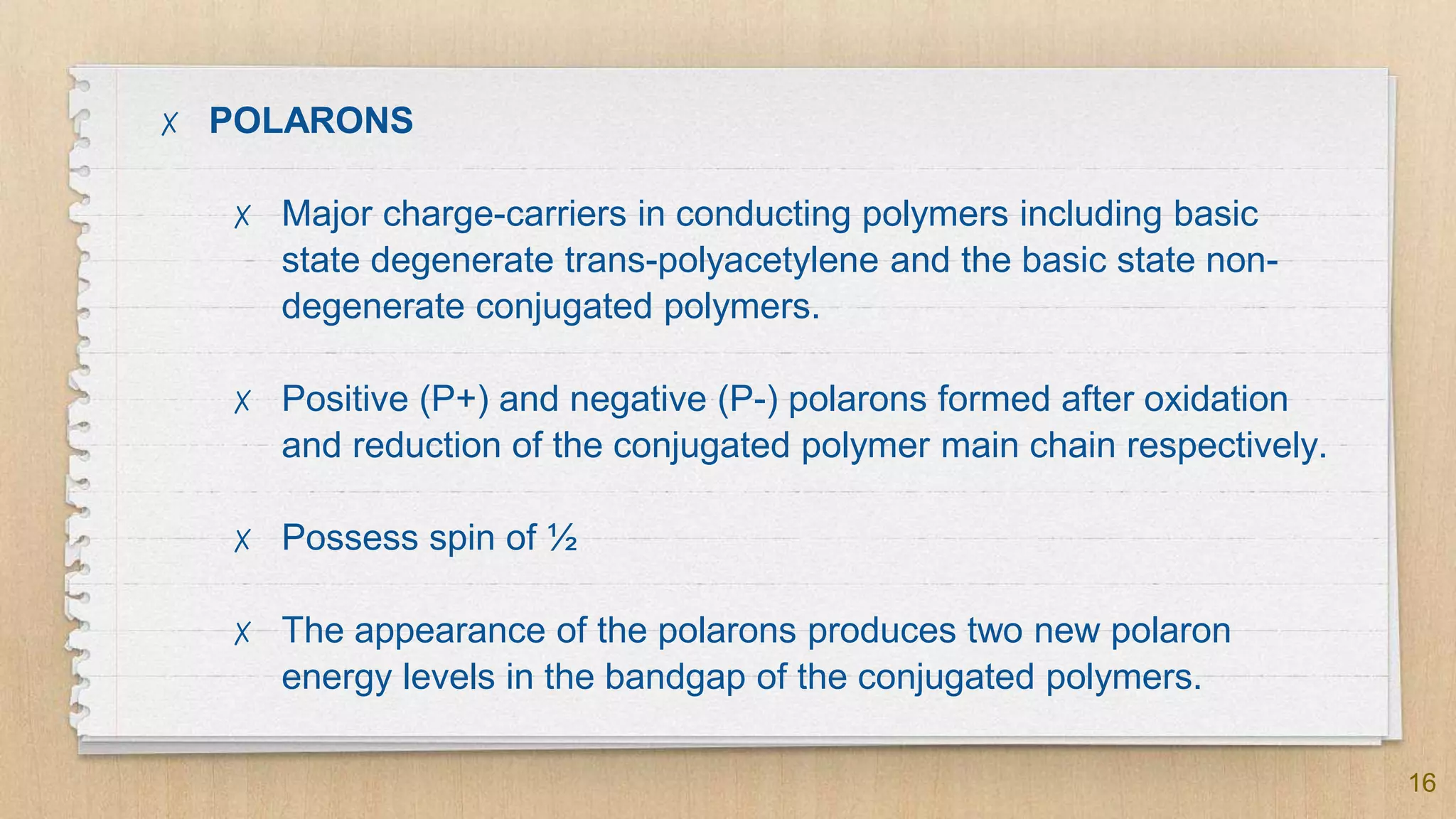
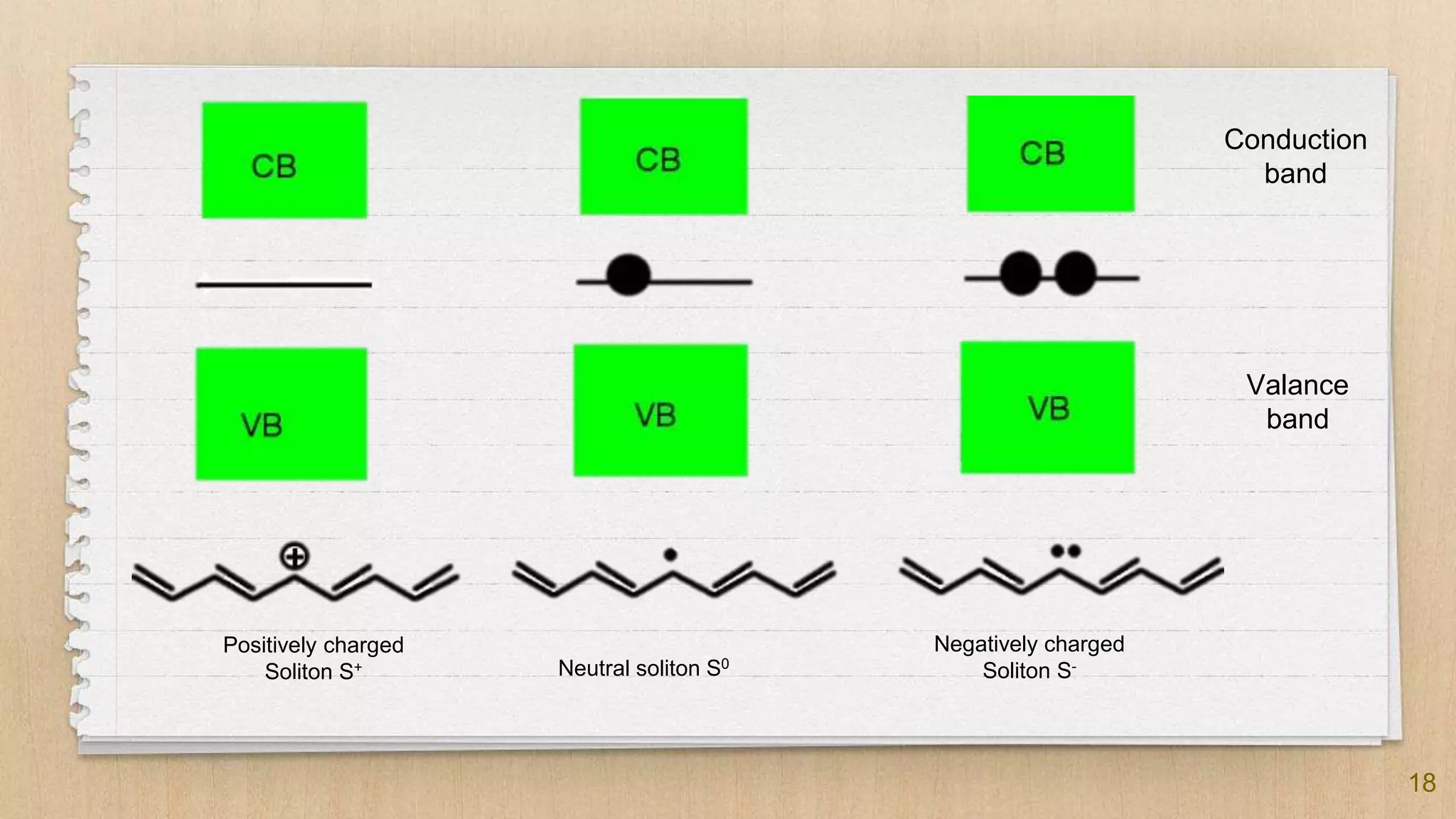
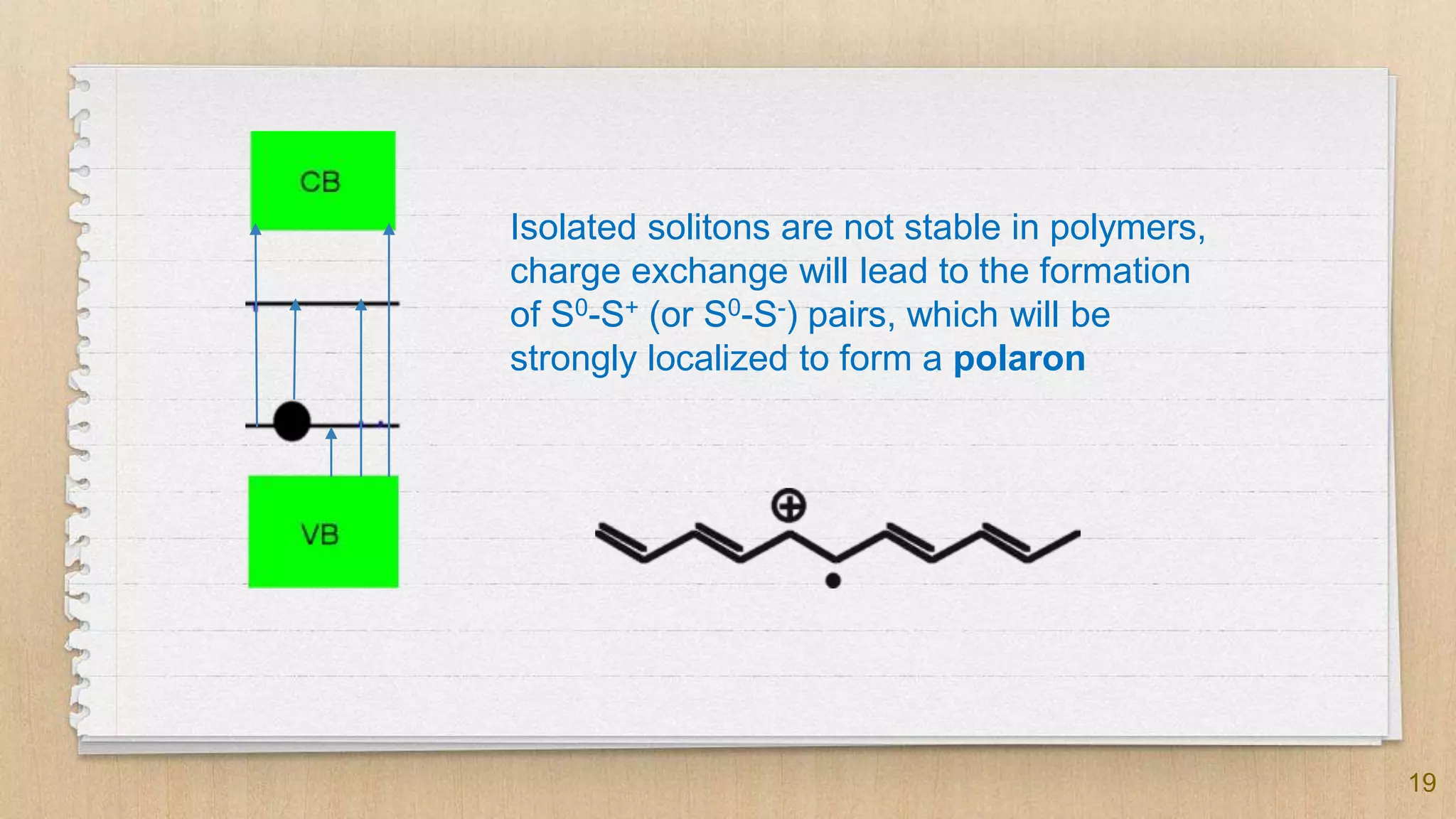
The seminar presented by Abhinav S on September 17, 2019, discusses the journey of conducting polymers from laboratory curiosity to commercial applications. It highlights the fundamental properties of polymers, factors affecting conductivity, and mechanisms for achieving conductivity in these materials, particularly focusing on polyacetylene. Conducting polymers are now utilized in various applications such as batteries, transistors, and biosensors, demonstrating the interdisciplinary expertise required for their development and market introduction.














![29
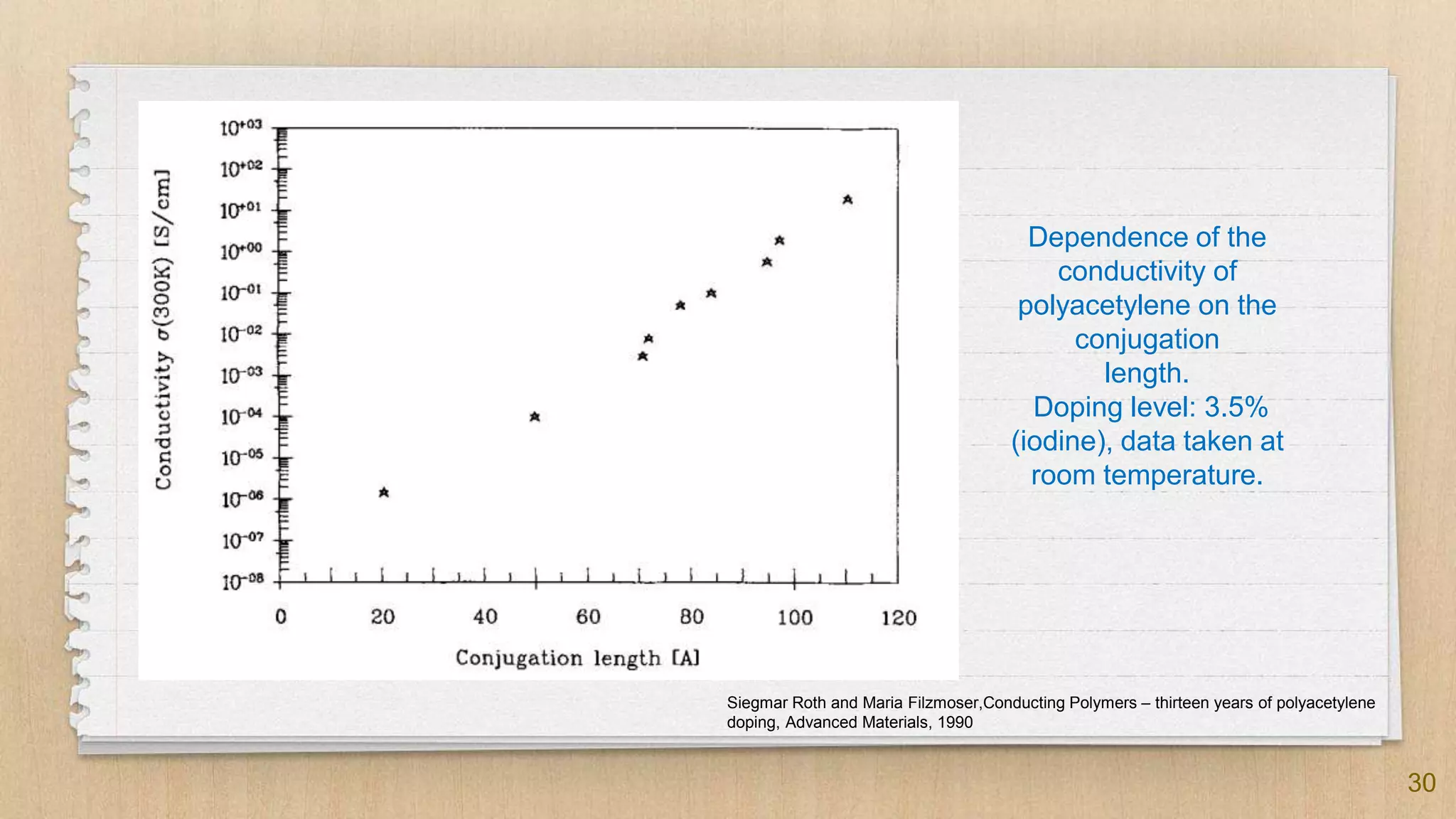
✗ The conductivity shows a temperature dependence similar to that of
semiconductors
✗ Obeys the Mott Variable Range Hopping (VRH) model:
✗ 𝜎(T) = 𝜎0 exp [ - (T0 / T)1/(n+1) ]
✗ n is dimension number
✗ 𝜎0 is a factor weakly related to temperature
✗ Conductivity closely related to the doping degree and the degree of
ordering of the polymer main chain
✗ The doping degree relates to the charge carrier concentration](https://image.slidesharecdn.com/seminarconductingpolymers-190925152603/75/Conducting-Polymers-29-2048.jpg)