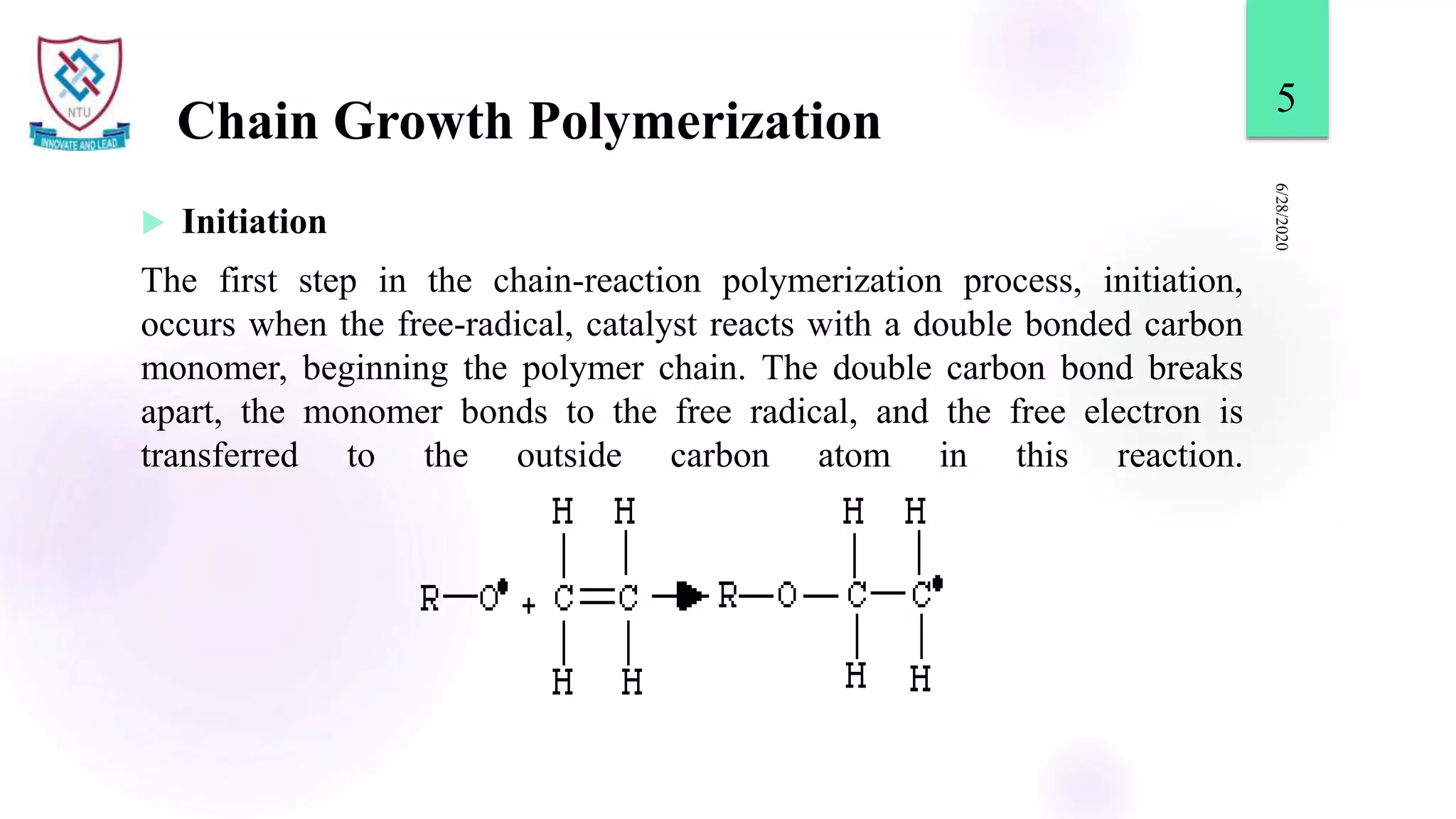
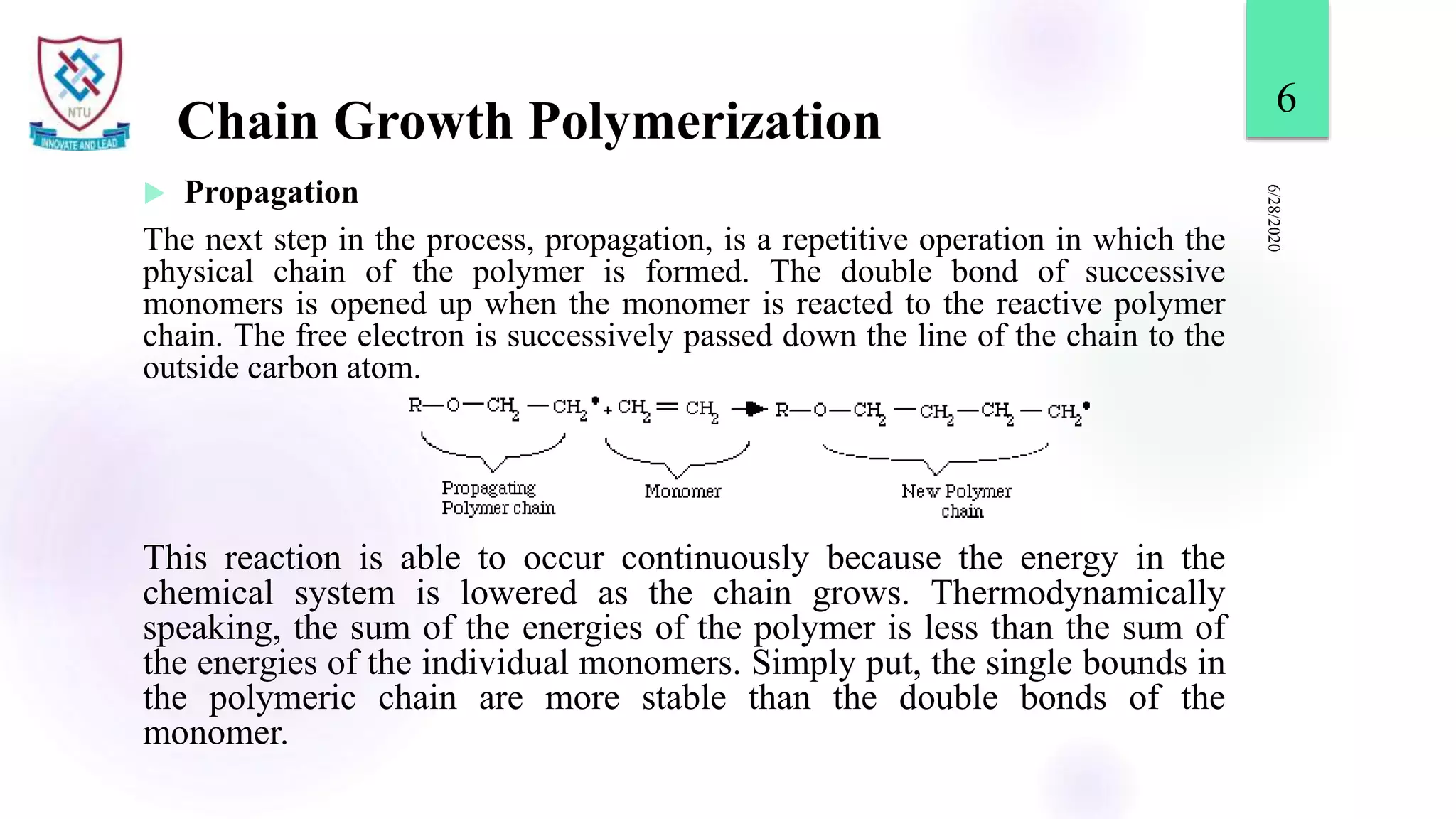
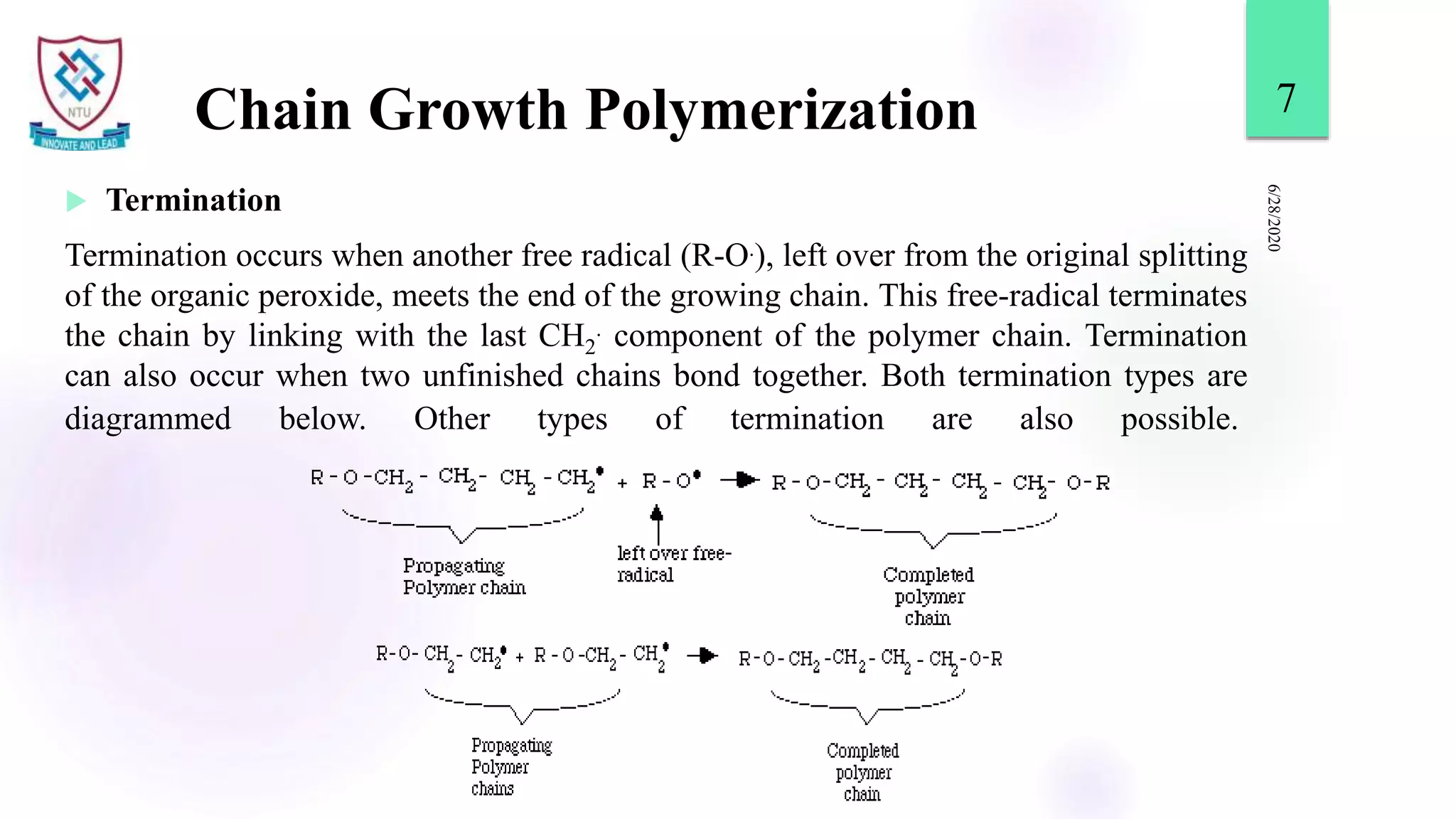
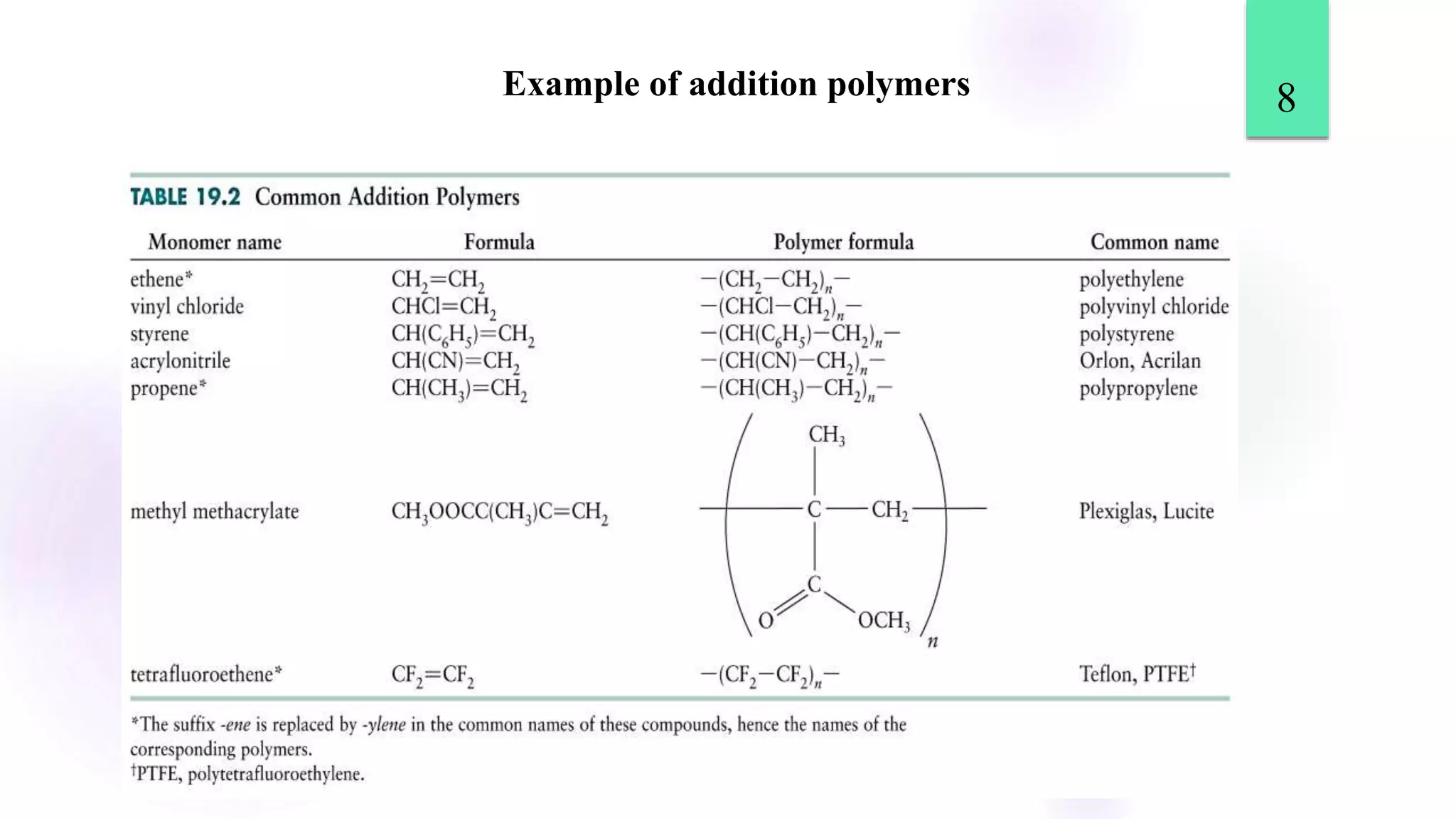
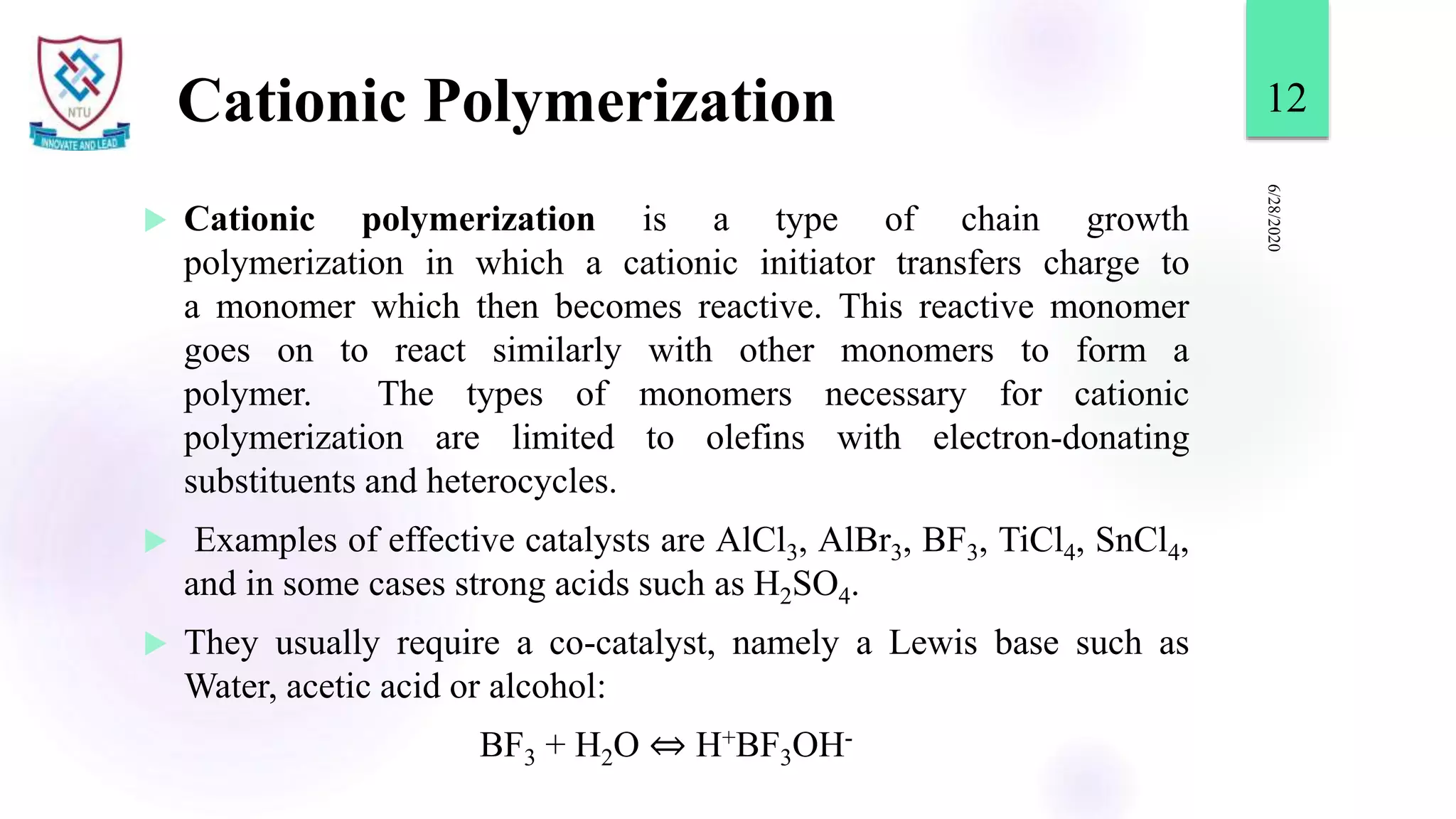
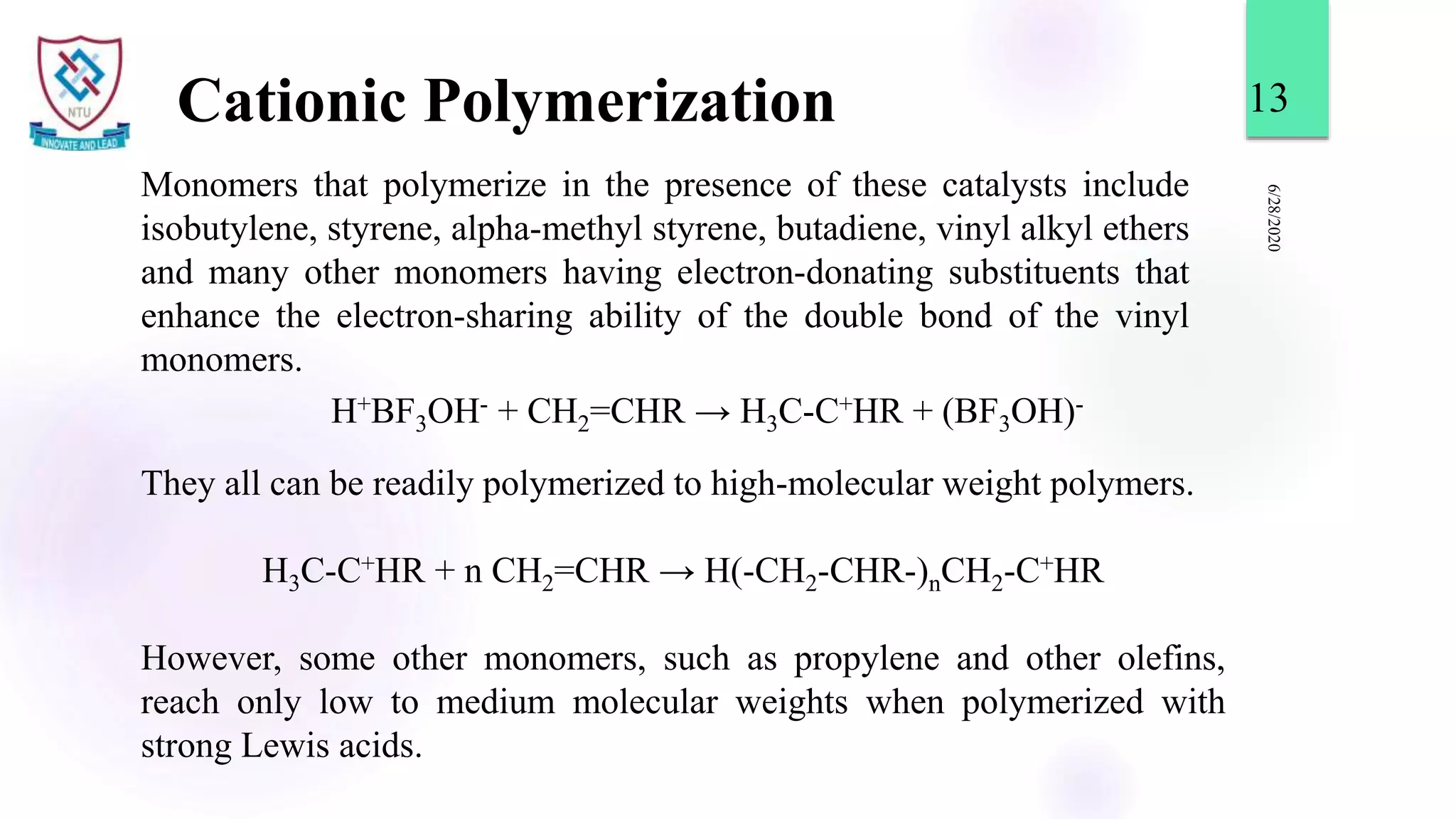
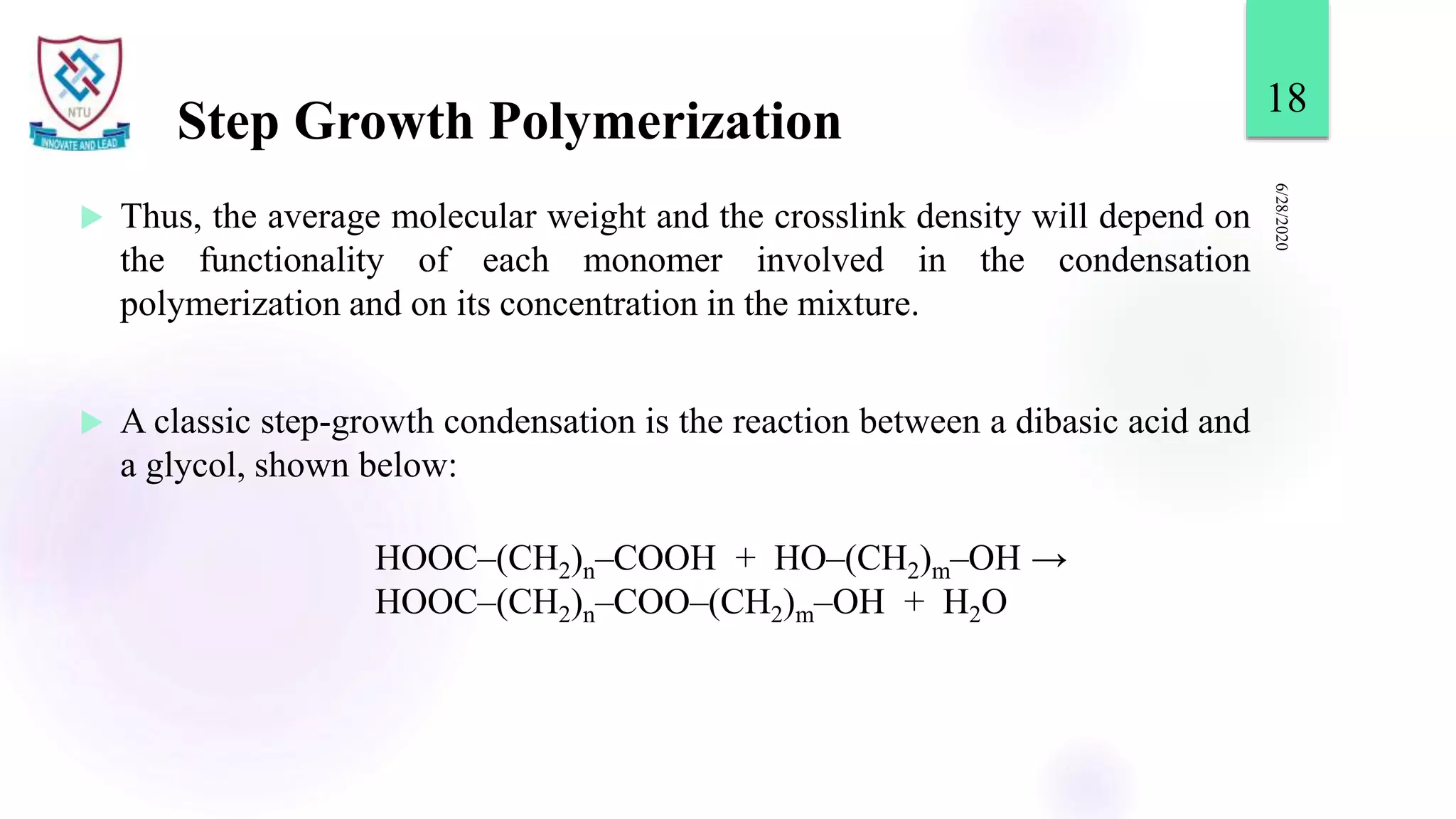
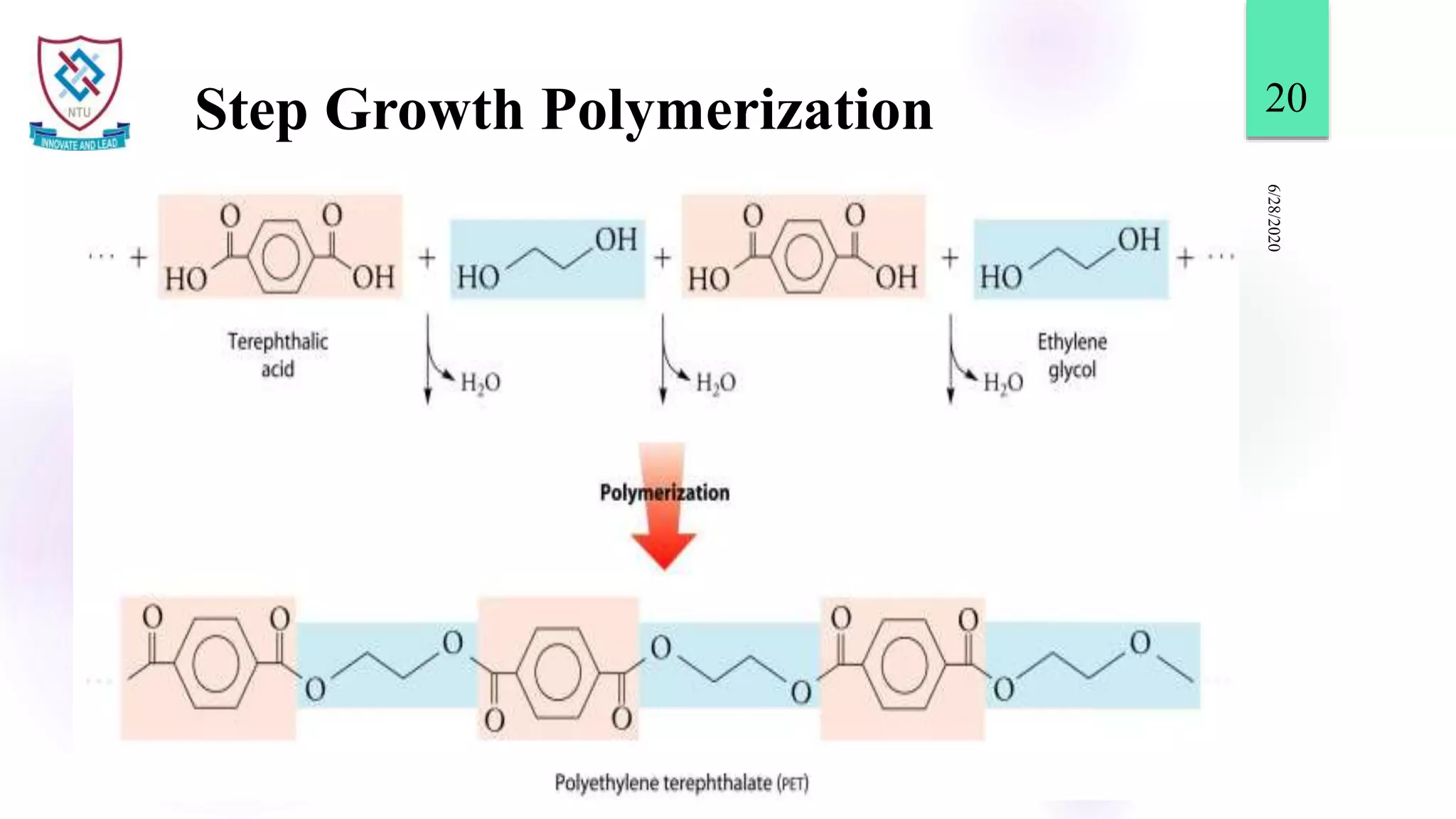
This document discusses different types of polymerization reactions including chain growth polymerization, step growth polymerization, and ionic polymerization. Chain growth polymerization involves initiation, propagation, and termination steps. Step growth polymerization involves condensation reactions between monomers to form polymers and byproducts like water. Ionic polymerization includes anionic polymerization using nucleophilic initiators and cationic polymerization using Lewis acid catalysts. Ziegler-Natta catalysis uses transition metal catalysts to polymerize monomers like propylene.