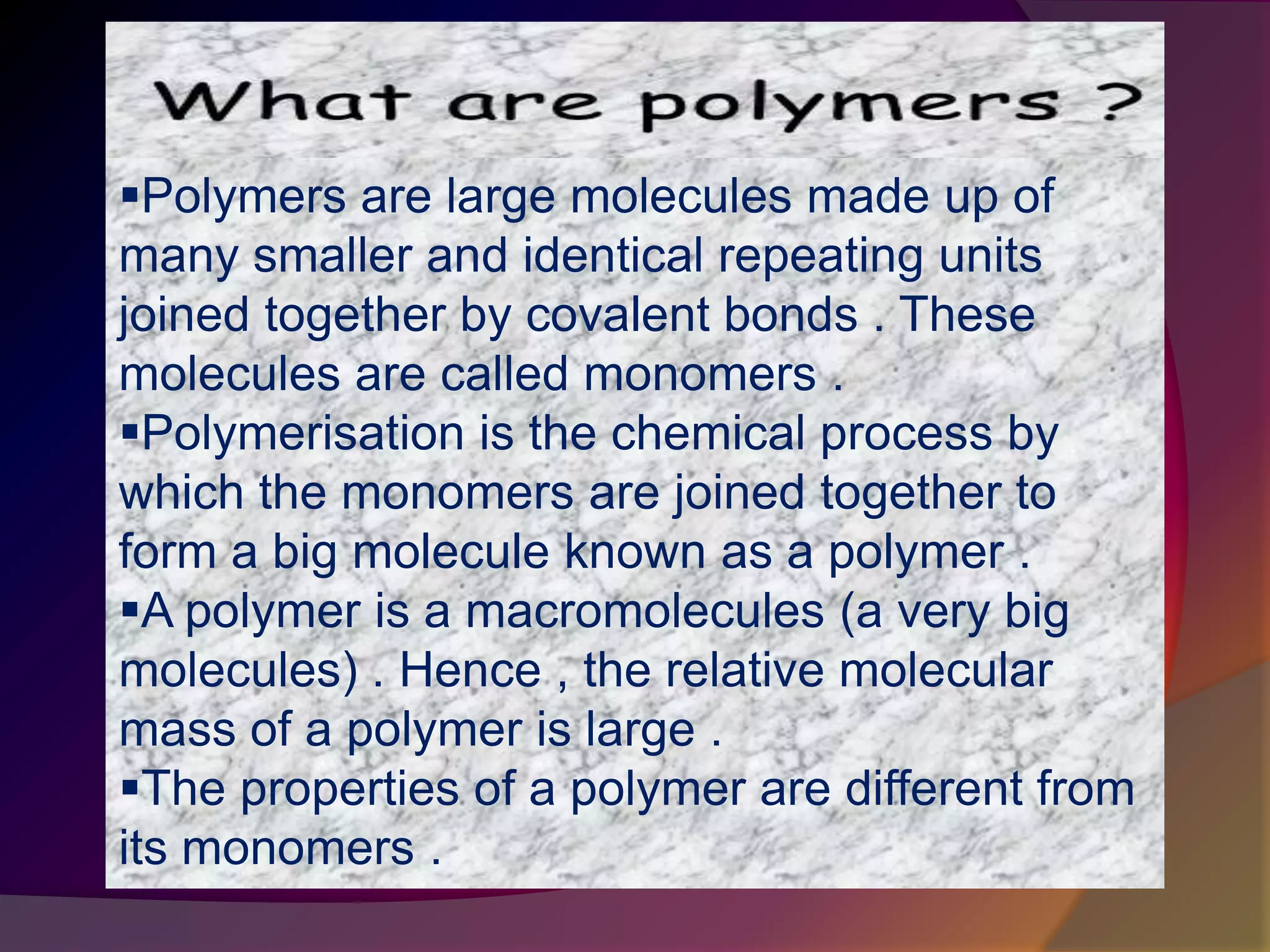
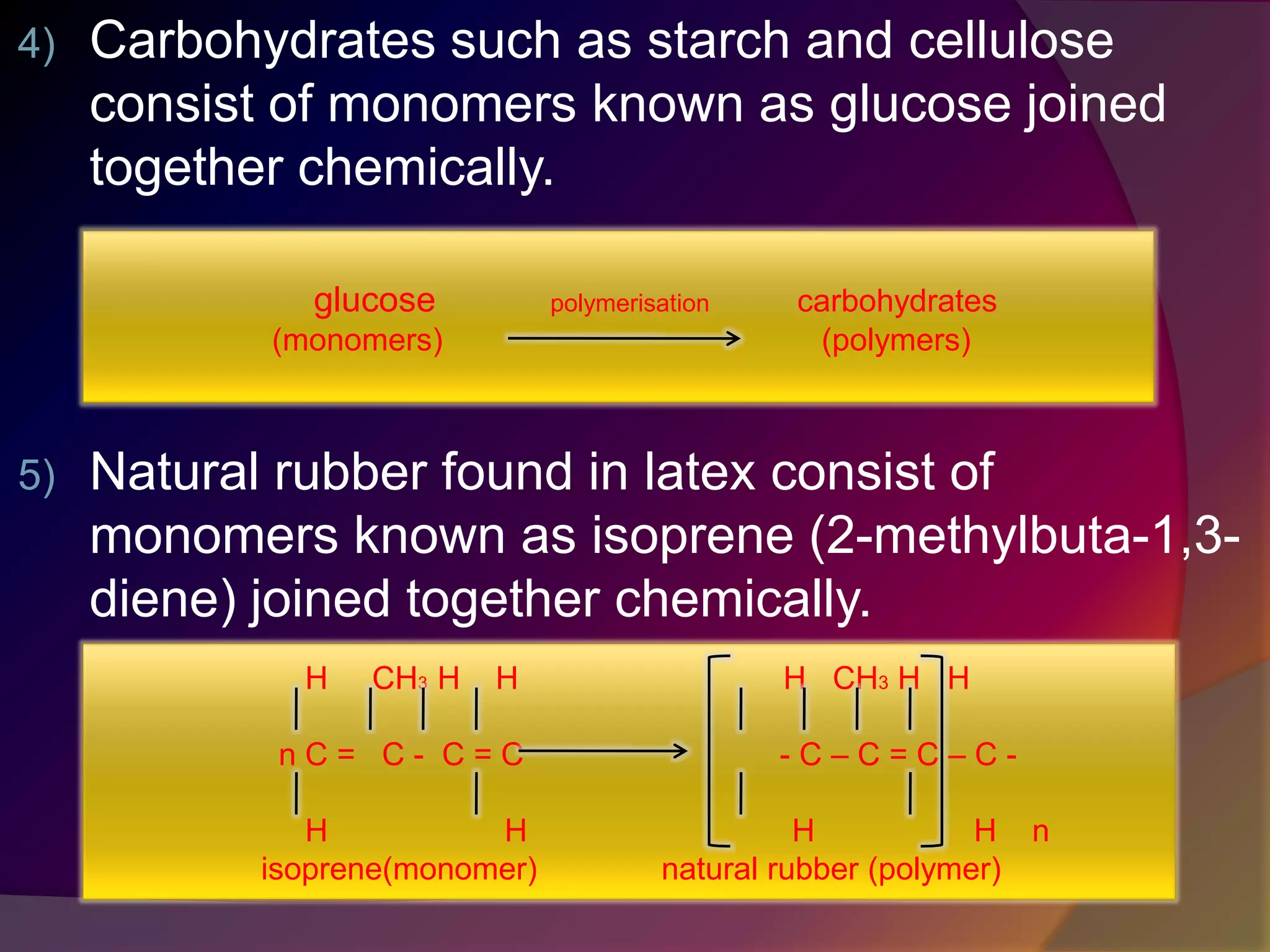
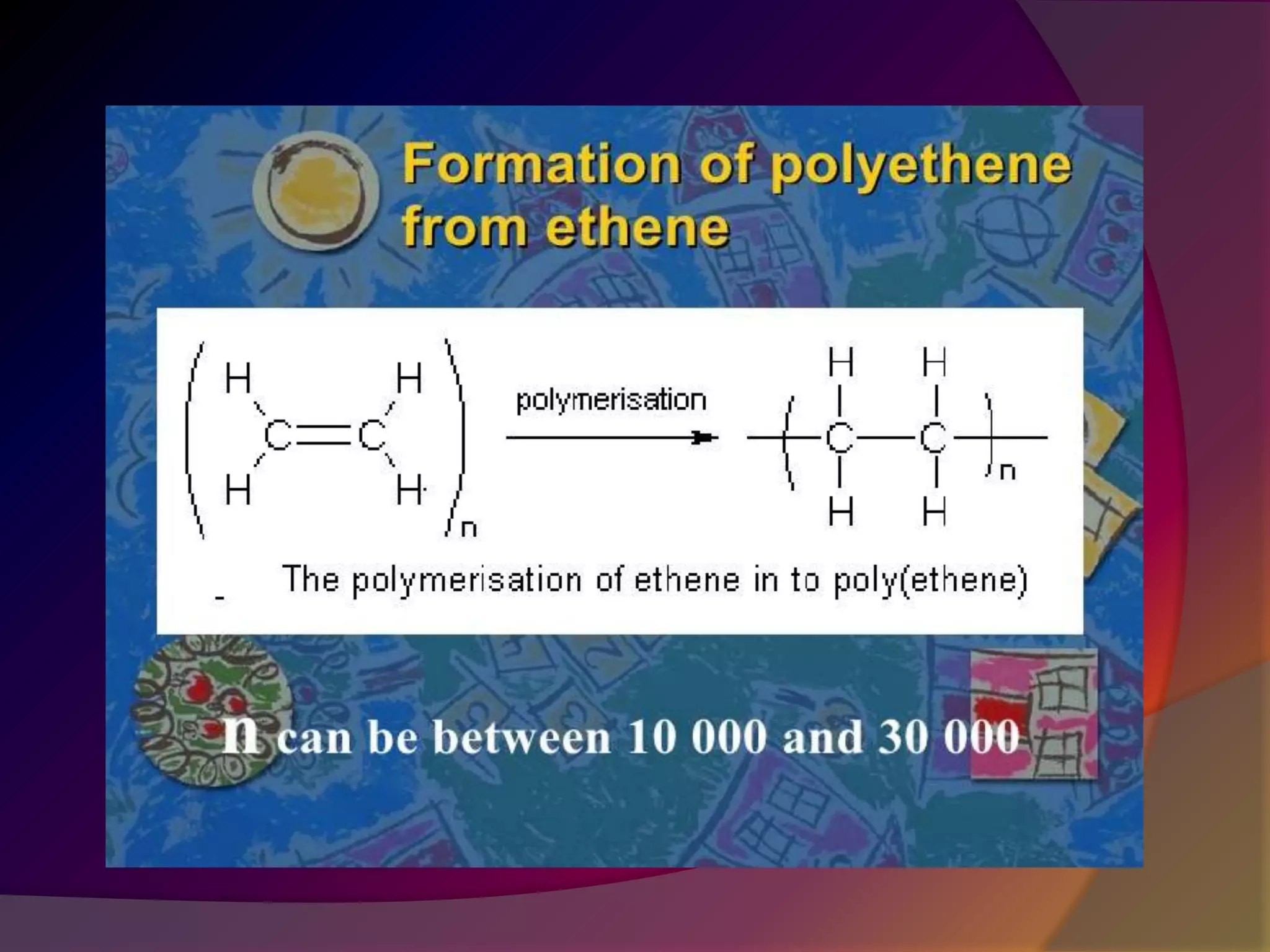
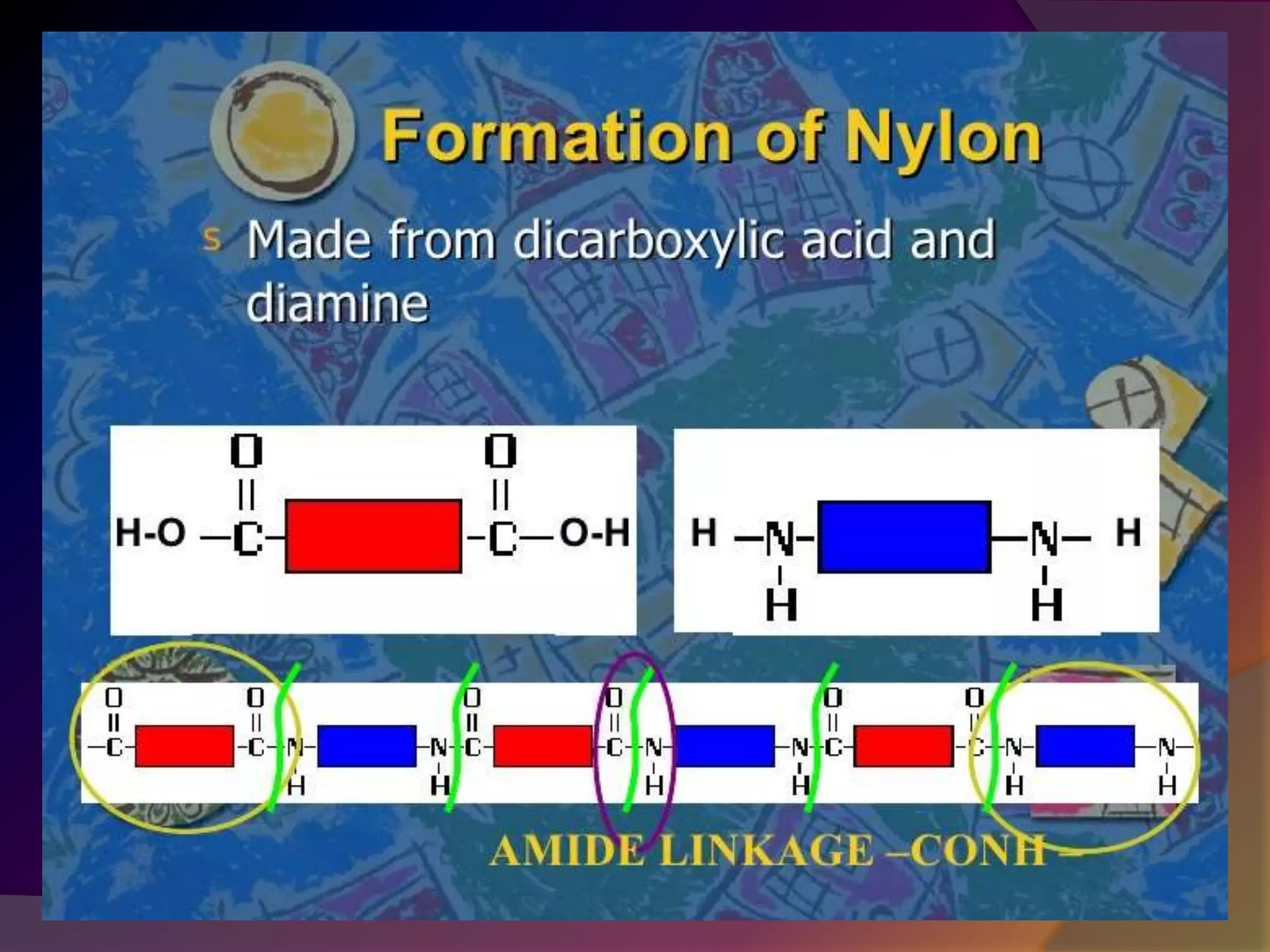
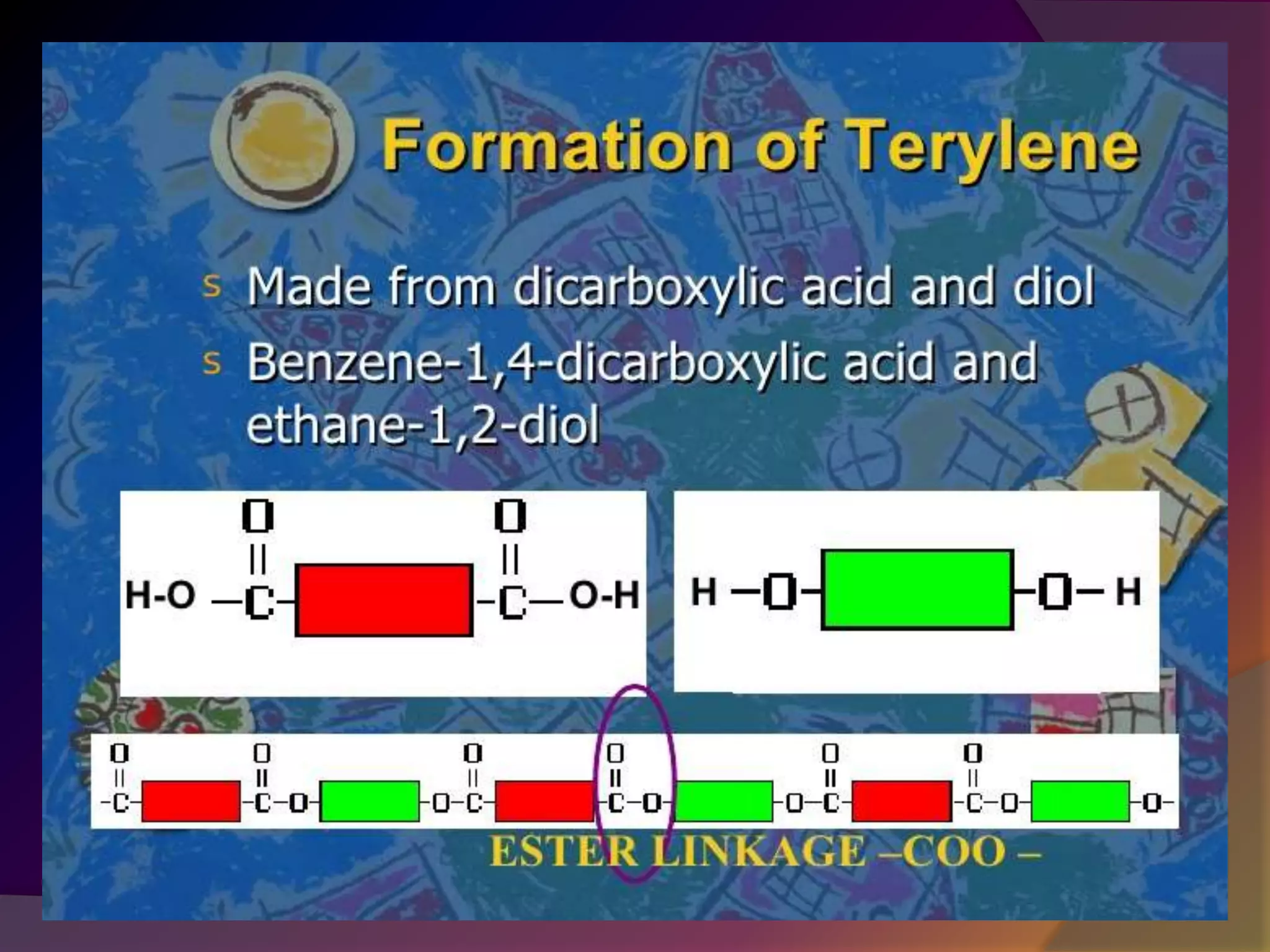
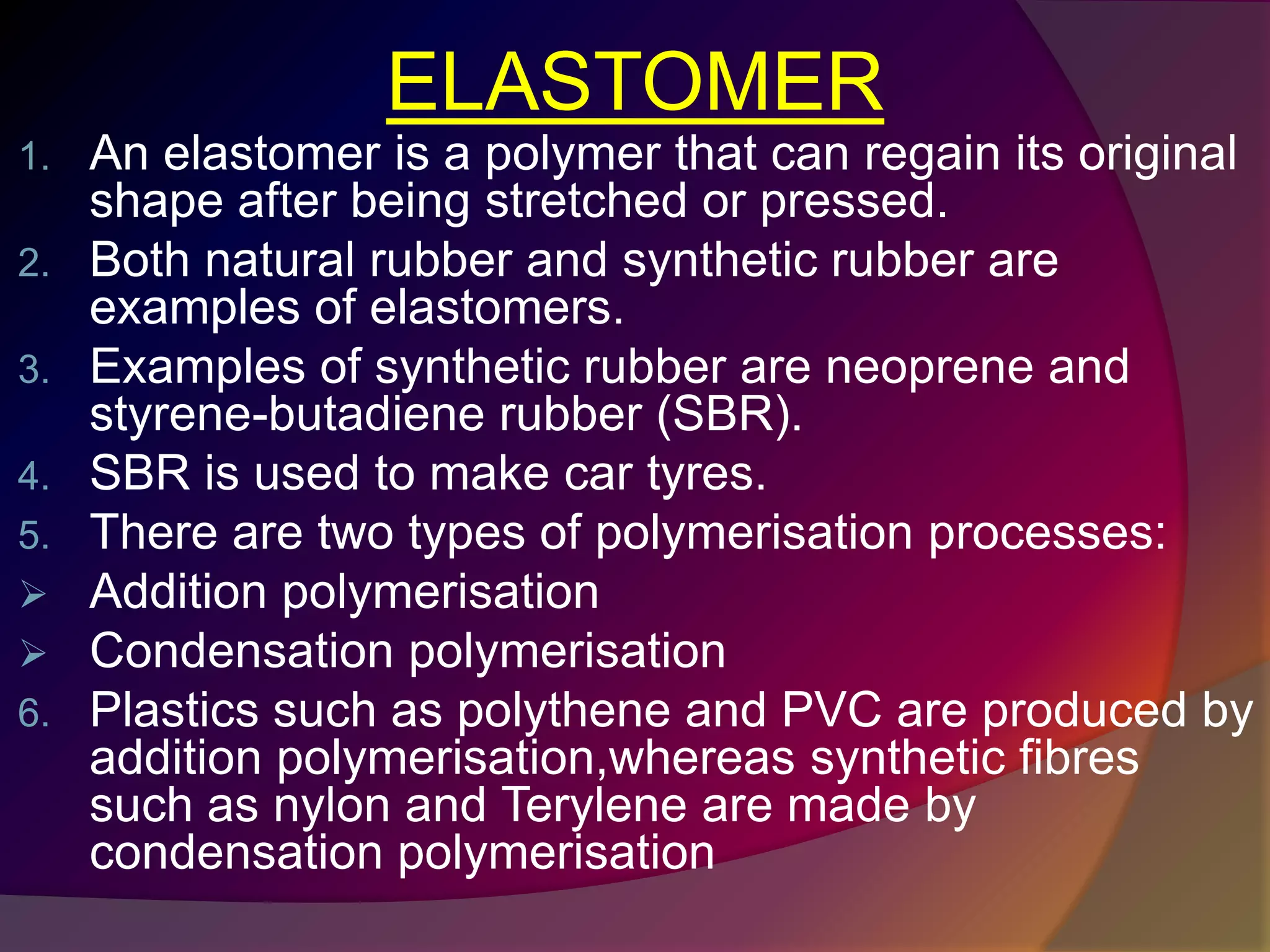
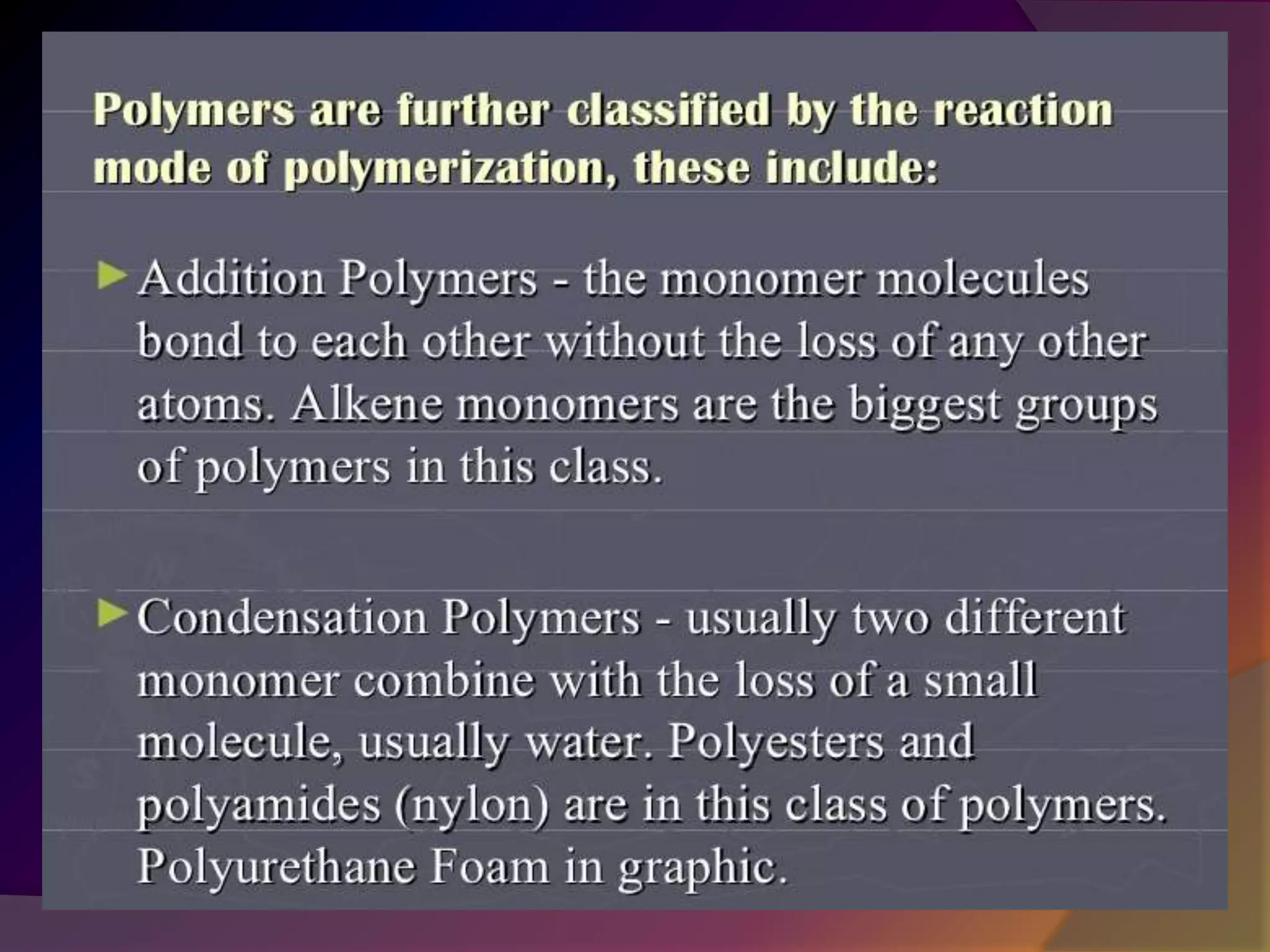
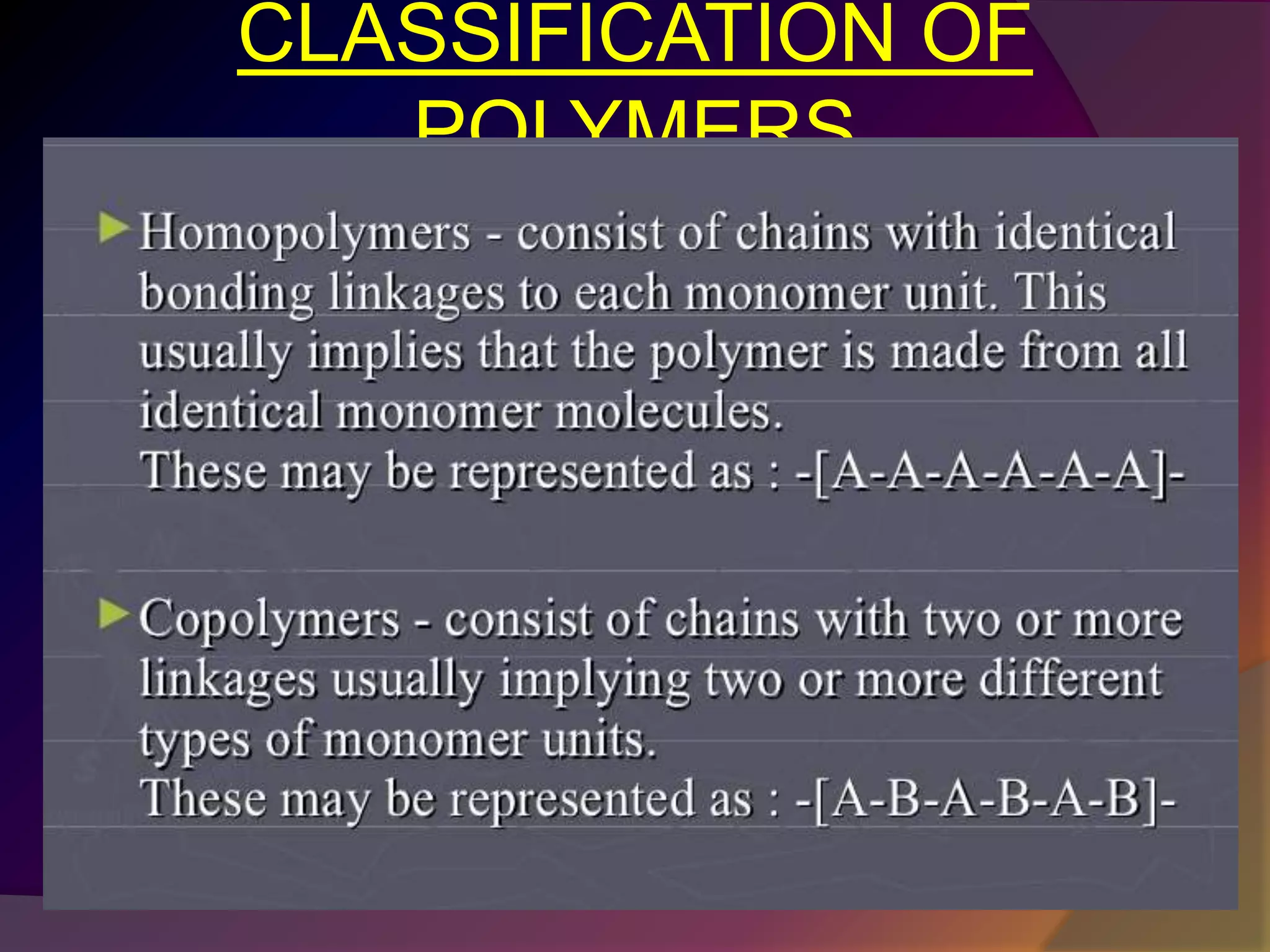
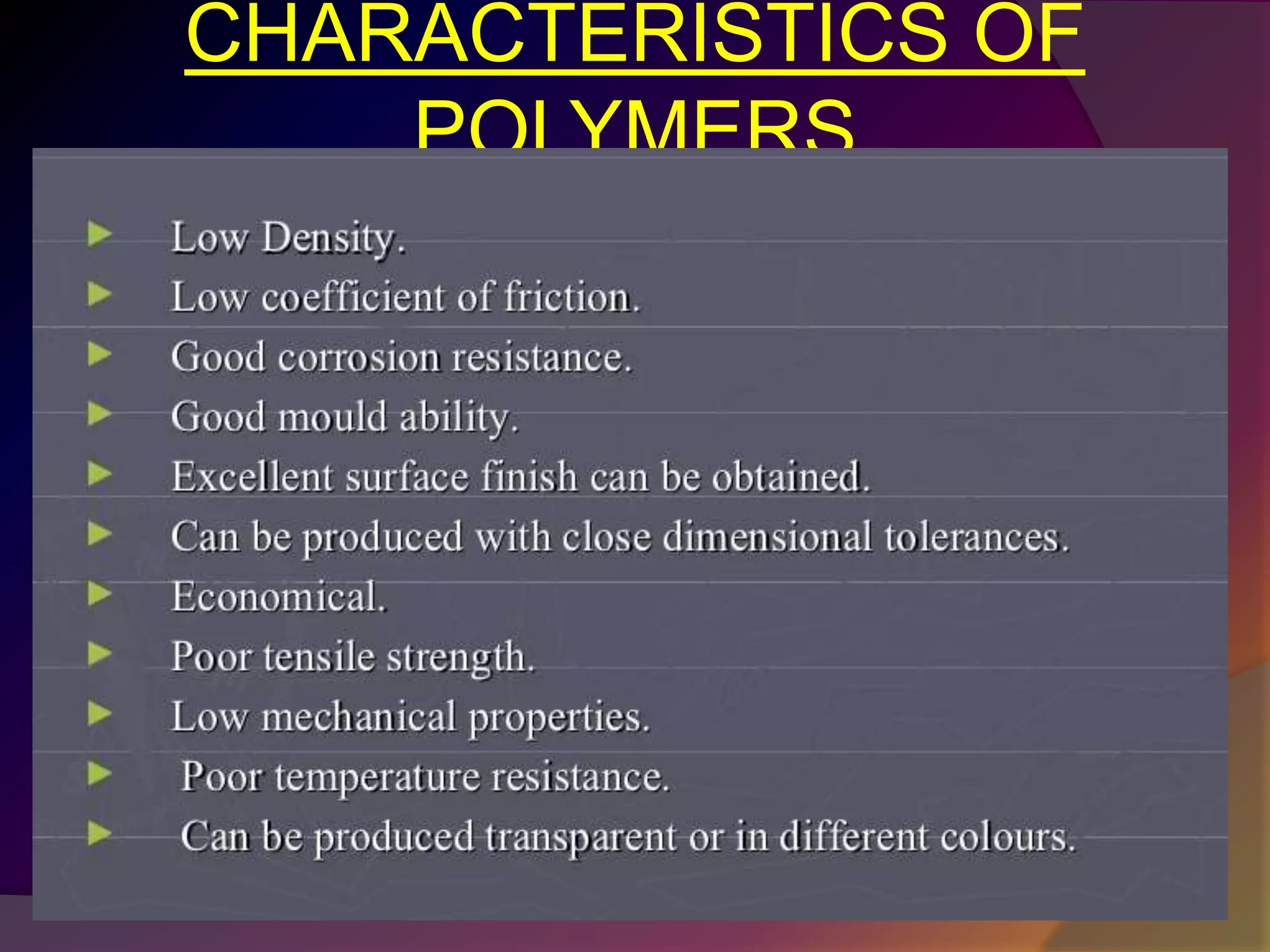
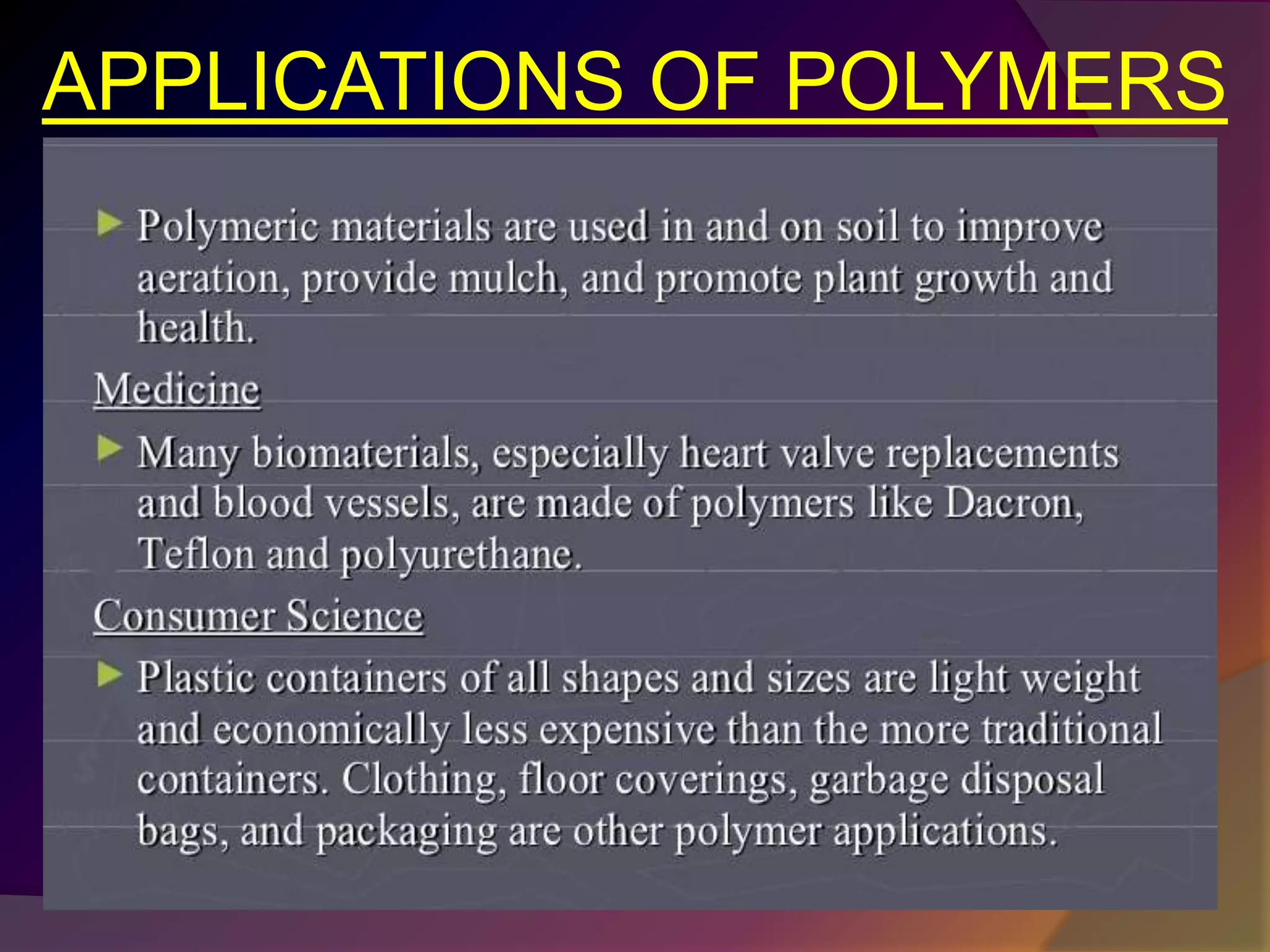
This document discusses synthetic polymers. Synthetic polymers are man-made polymers created through chemical processes in laboratories by linking monomers together. Examples of synthetic polymers include plastics, fibers, and elastomers. While synthetic polymers have many applications, they also cause pollution problems as most are non-biodegradable. Methods to address this include reducing use, reusing and recycling polymers, as well as developing biodegradable polymers.