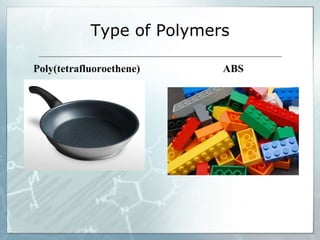
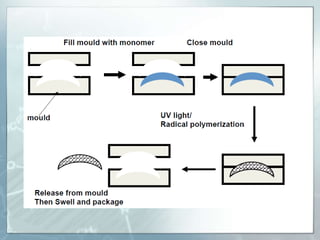
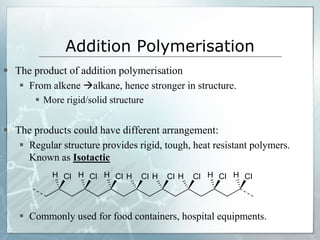
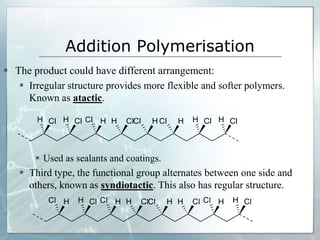
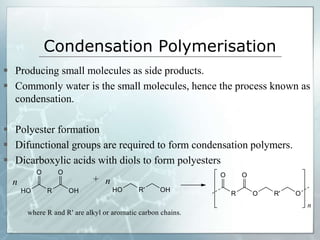
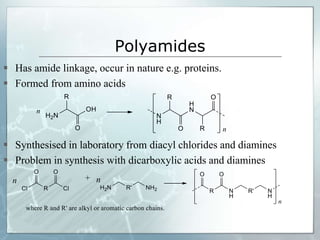
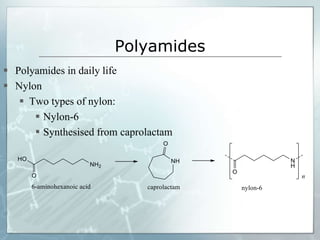
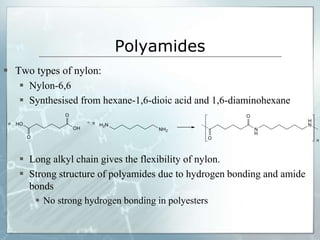
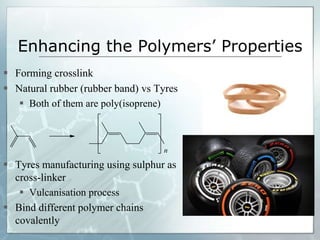
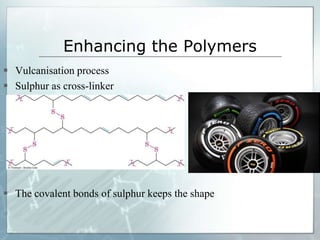
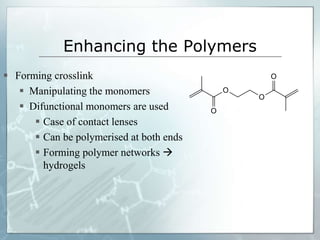
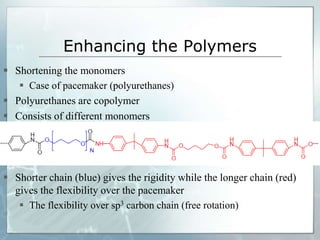
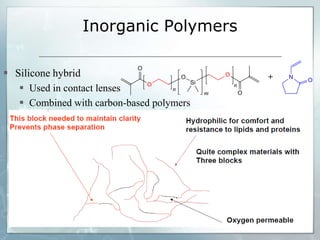
The document provides an overview of polymer chemistry, defining polymers as macromolecules made from monomers and detailing their types, such as homopolymers and copolymers. It discusses synthesis methods like addition and condensation polymerization, including examples like PVC and polyesters, as well as the properties and applications of various synthetic polymers in daily life. Additionally, the document covers property enhancement techniques for polymers, including using cross-linkers and varying monomer chains.