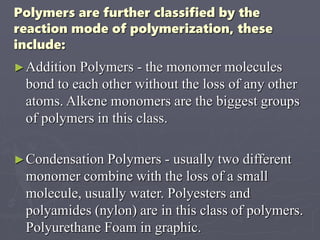
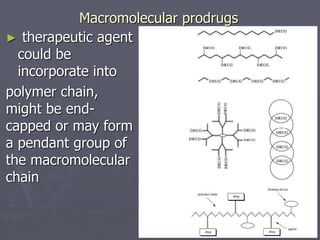
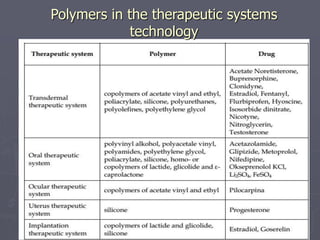
This document discusses polymers, including their classification, characteristics, properties, strengths, and applications. It begins with an introduction to polymers being long chains of repeating monomer units. It then covers the main topics of types of polymers like polyethene and nylon, how they are classified based on composition and reaction, their characteristics like low density and good moldability. Properties depend on chain length and structure. Strength increases with longer chains and higher crystallinity. Finally, applications of polymers in areas like packaging, textiles, construction, medicine and pharmaceuticals are outlined.






![Classification of Polymers
►Homopolymers - consist of chains with identical
bonding linkages to each monomer unit. This
usually implies that the polymer is made from all
identical monomer molecules.
These may be represented as : -[A-A-A-A-A-A]-
►Copolymers - consist of chains with two or more
linkages usually implying two or more different
types of monomer units.
These may be represented as : -[A-B-A-B-A-B]-](https://image.slidesharecdn.com/1-220929055832-0177f7aa/85/1-Polymer-ppt-8-320.jpg)