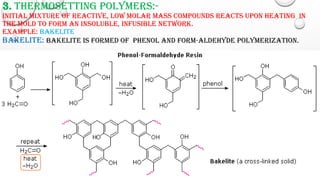
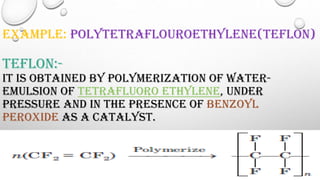
The document discusses polymers including their classification, types of polymerization, characteristics, and applications. Polymers can be classified based on source, structure, polymerization method, and molecular forces. There are two main types of polymerization - addition and condensation. Polymers have characteristics like low density, corrosion resistance, and moldability. They have wide applications in medicine, consumer products, industry, and sports equipment.






![CLASSIFICATION BASED ON
POLYMERIZATION
1. ADDITION POLYMERS
FORMED BY THE REPEATED ADDITION OF MONOMER MOLECULES POSSESSING DOUBLE OR TRIPLE
BONDS
N(CH2=CH2) -(CH2 -CH2 )-
ETHYLENE POLYETHYLENE
2. CONDENSATION POLYMERS
FORMED BY REPEATED CONDENSATION REACTION BETWEEN TWO DIFFERENT BI-FUNCTIONAL OR TRI-
FUNCTIONAL MONOMERIC UNITS.
EG. TERYLENE (DACRON), NYLON 6, 6, NYLON 6.
N(H2N(CH2)6 NH2) + N(HOOC(CH2)4COOH) [-NH(CH2)6NHCO(CH2)4CO-]N + NH2O
(NYLON 6:6)](https://image.slidesharecdn.com/polymerppt-140425171028-phpapp02/85/Polymer-ppt-7-320.jpg)
![CLASSIFICATION BASED ON MOLECULER FORCE
1. NYLON :- NYLON IS USED AS GENERAL NAME FOR ALL SYNTHETIC FIBER FORMING
POLYAMIDES,I.E., HAVING A PROTEIN LIKE STRUCTURE. THESE ARE THE
CONDENSATION POLYMERS OF DIAMINES AND DIBASIC ACIDS A NUMBER IS
USUALLY SUFFIXED WITH THE NYLON WHICH REFERS TO THE NUMBER OF CARBON
ATOMS PRESENT IN THE DIAMINE AND THE DIBASIC ACIDS RESPECTIVELY.
EXAMPLE: NYLON 6,6
NYLON-6,6: NYLON-6,6 IS OBTAINED BY THE POLYMERISATION OF ADIPIC ACID
WITH HEXAMETHYLENE DIAMINE.
nHOOC(CH2)4COOH + nH2N(CH2)6 NH2
553K [-N-(CH2) 6-N- C(CH2)4-C-]n
High pressure
H O O](https://image.slidesharecdn.com/polymerppt-140425171028-phpapp02/85/Polymer-ppt-8-320.jpg)