The document discusses various topics related to polymerization including:
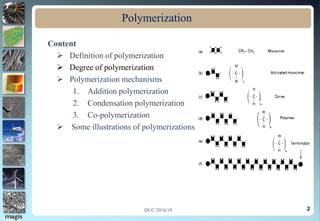
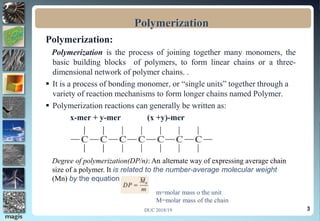
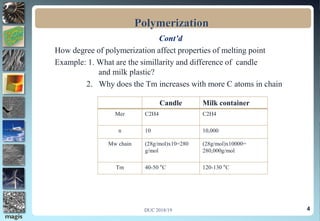
1. Definitions of polymerization, degree of polymerization, and different polymerization mechanisms including addition, condensation, and co-polymerization.
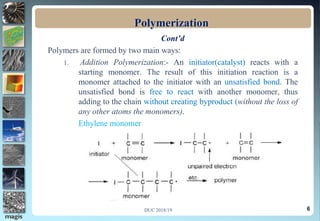
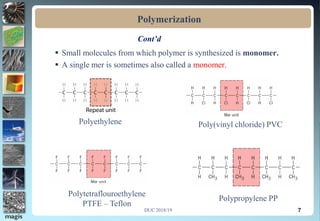
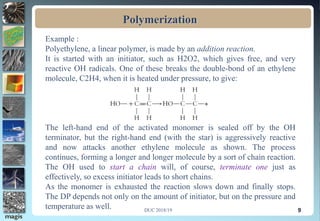
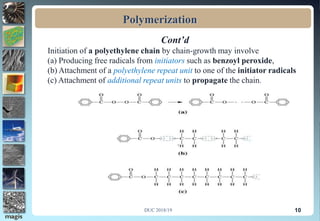
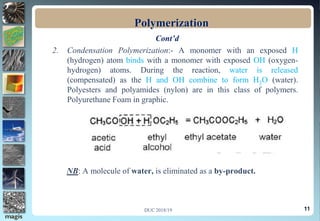
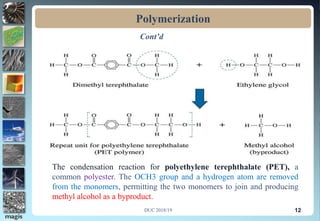
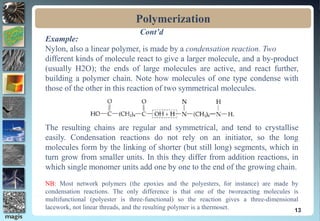
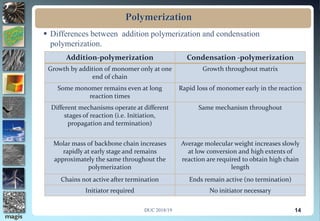
2. Addition polymerization involves monomers adding to the growing chain without byproducts, while condensation polymerization eliminates molecules like water as monomers join.
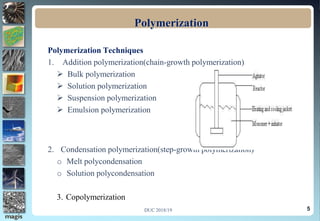
3. Common polymerization techniques are discussed briefly, including bulk, solution, suspension, and emulsion polymerization.