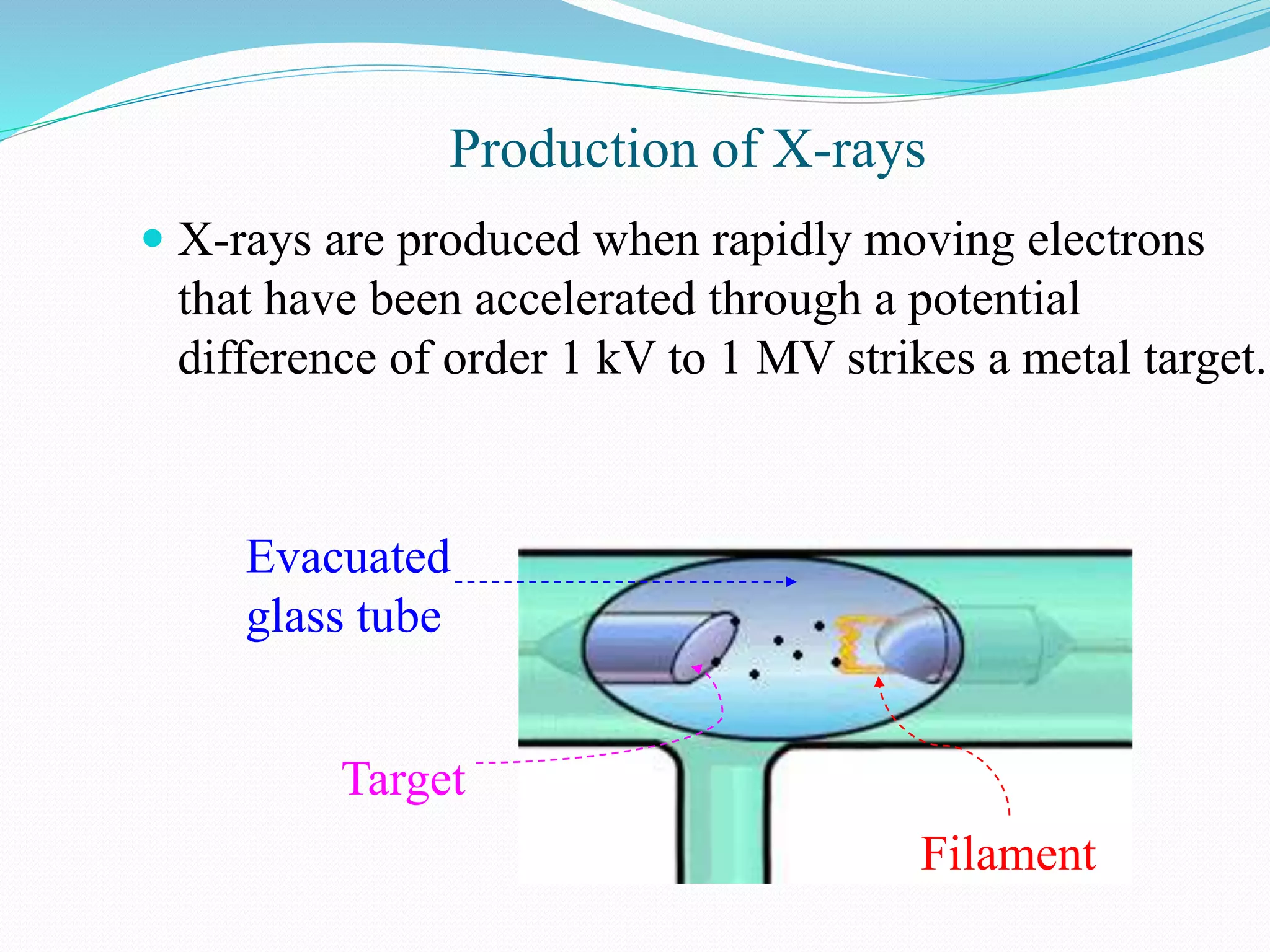
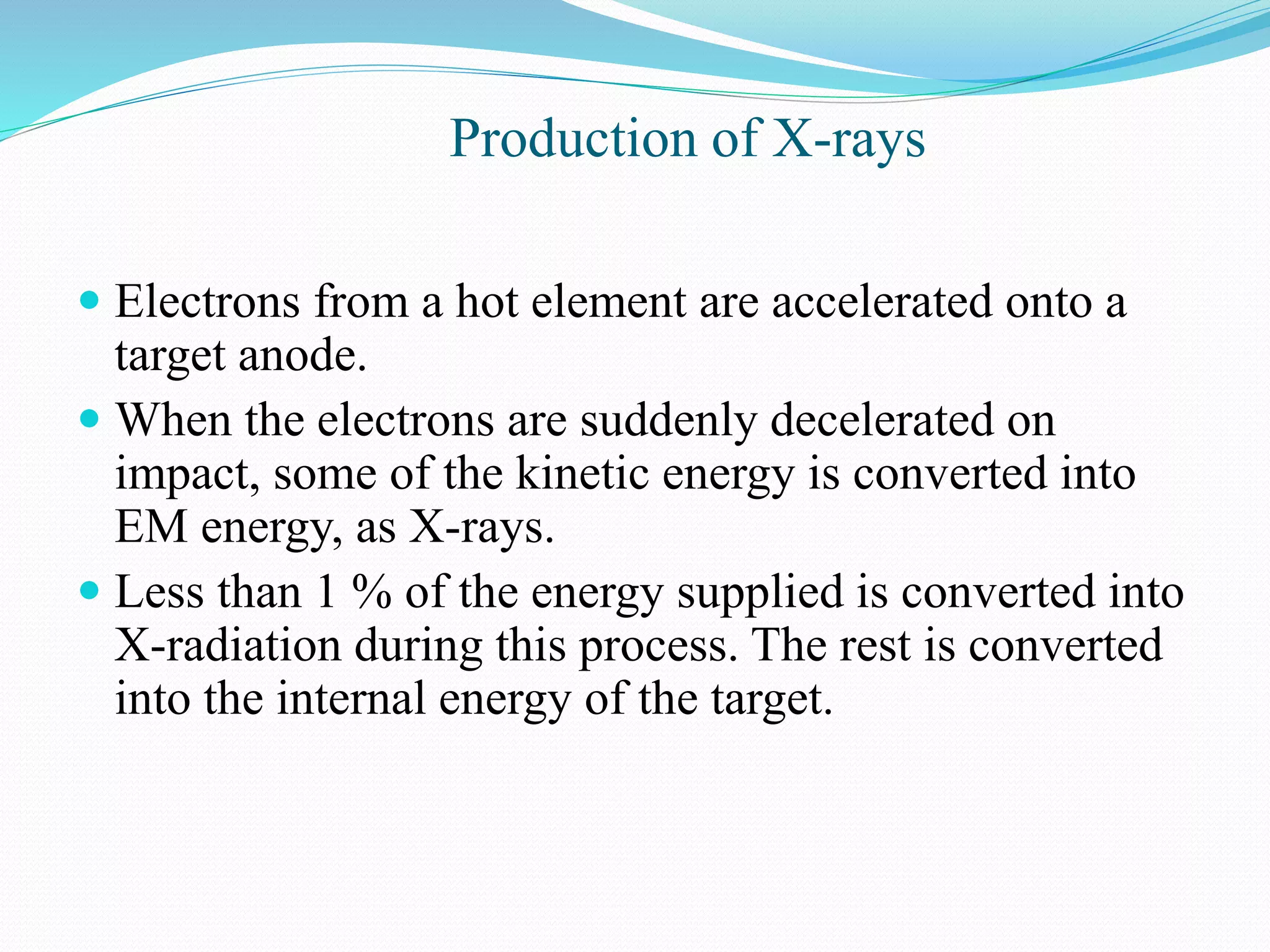
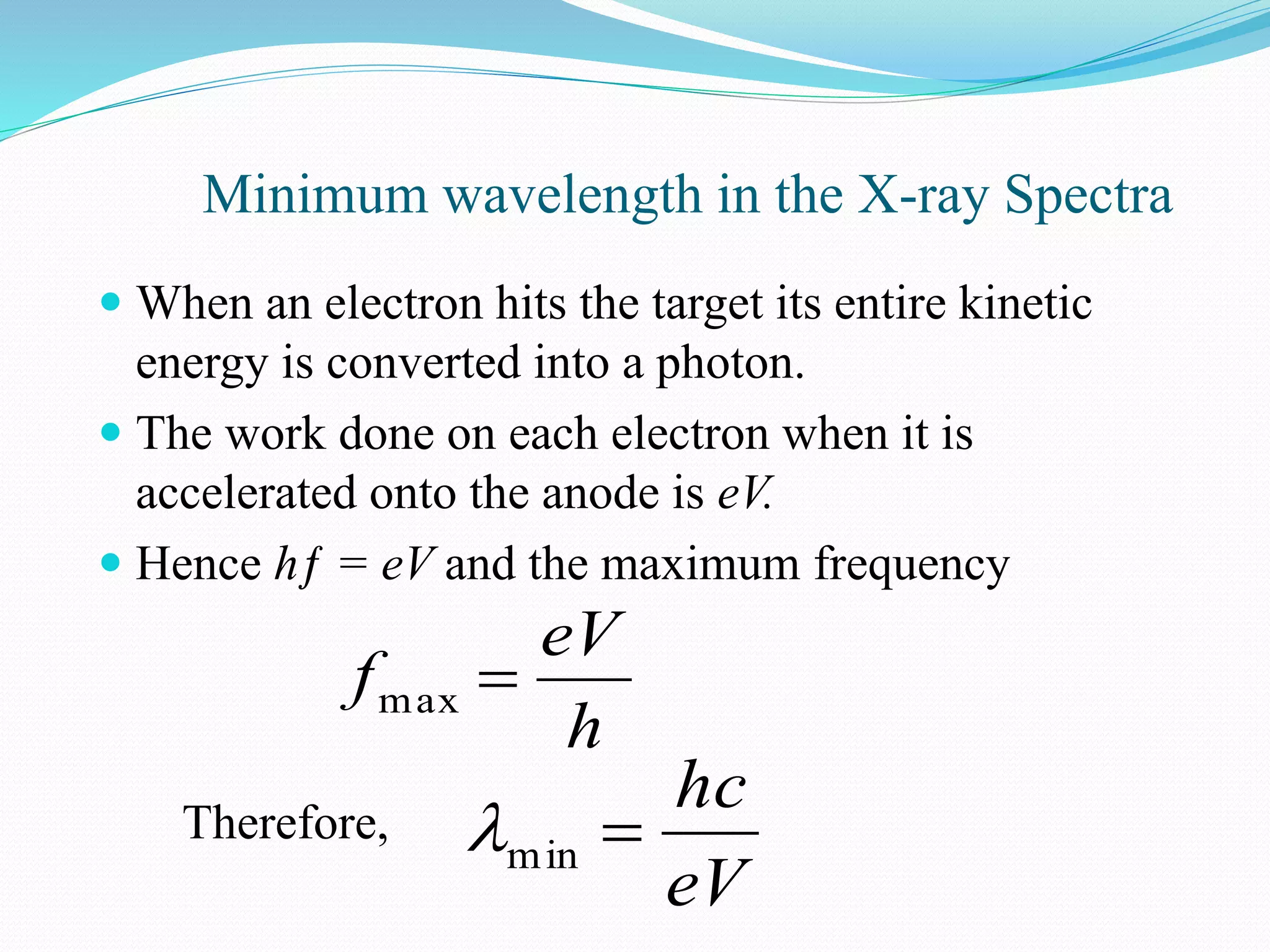
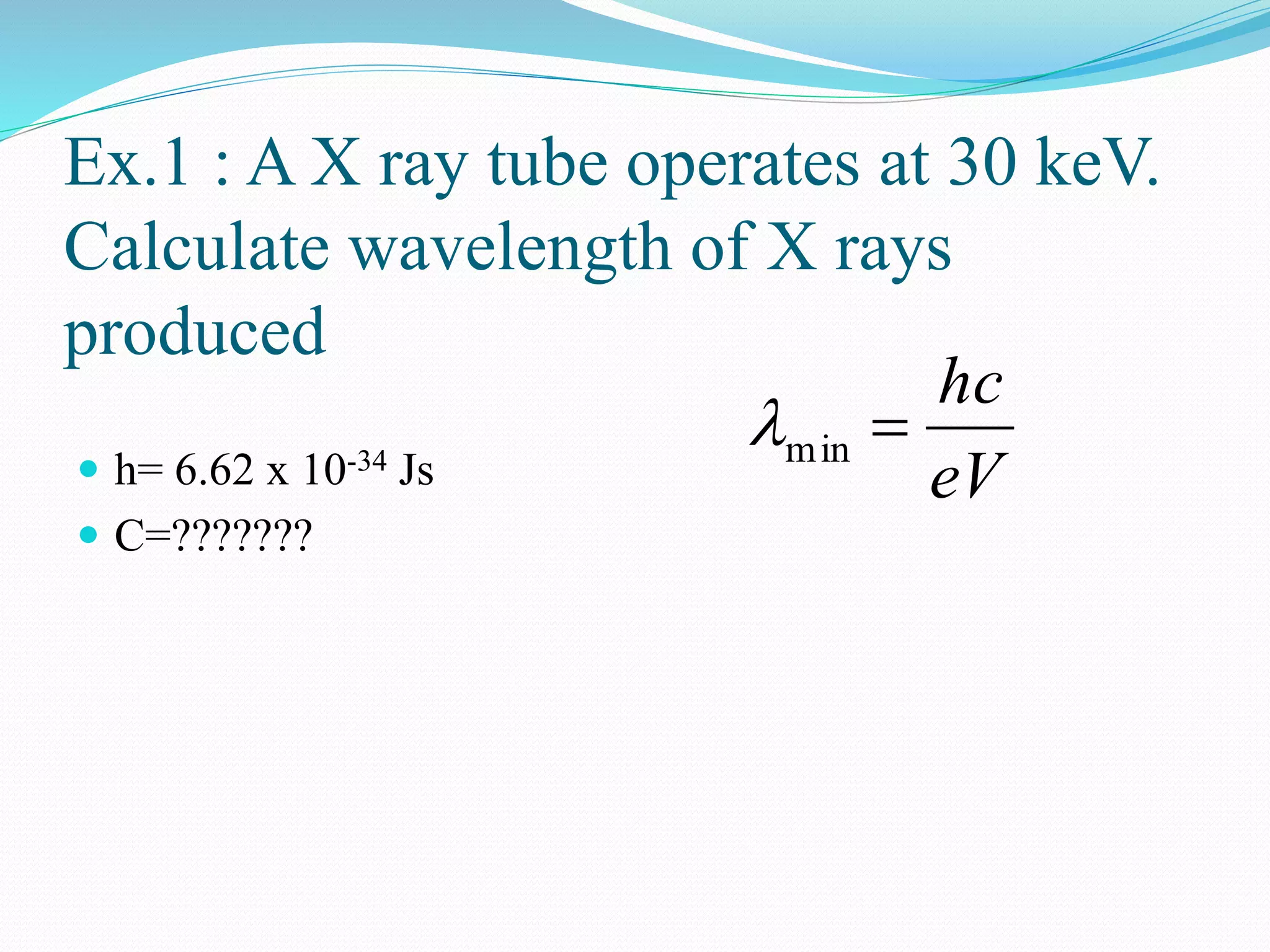
X-rays are produced when electrons accelerated to speeds of 1 kV to 1 MV strike a metal target. Less than 1% of the kinetic energy is converted to X-radiation, with the rest becoming heat in the target. Moseley's law states that the wavelength of characteristic X-rays is related to the atomic number by the formula 1/λ = c(Z - s)2, where c and s are constants and Z is the atomic number. This helped establish atomic number as more fundamental than atomic weight in determining an element's position in the periodic table. X-rays have various applications including medical imaging and material characterization.