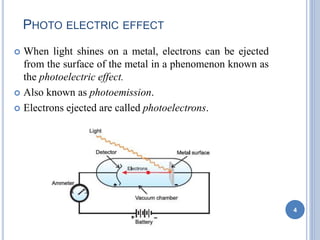
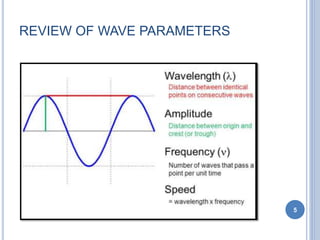
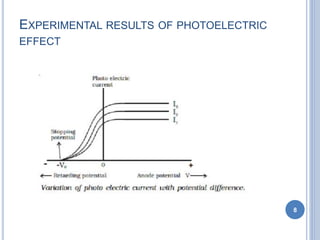
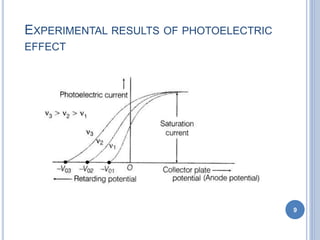
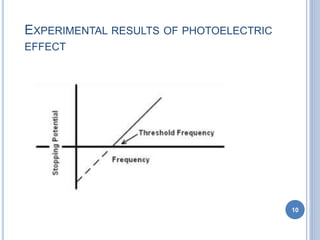
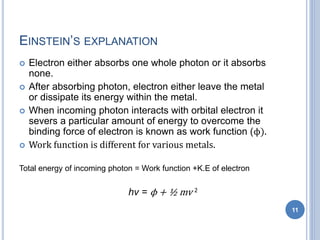
This document discusses the photoelectric effect and provides an overview of key concepts and experimental results. It introduces the photoelectric effect as the ejection of electrons from a metal surface when light shines on it. Experimental results showed that increasing light intensity increases the number of ejected electrons but not their velocity, and that there is a threshold frequency below which the effect does not occur. Einstein's explanation was that electrons absorb entire photons at once, and his photoelectric equation relates the photon's energy to the kinetic energy of the ejected electron.