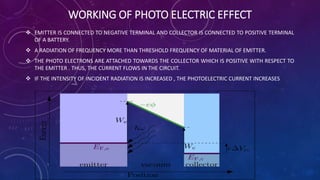
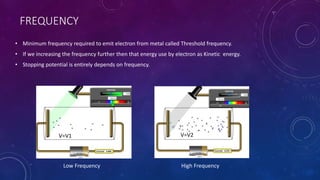
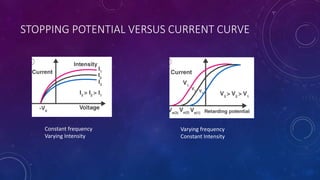
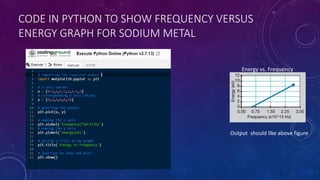
The photoelectric effect occurs when light irradiates a metal surface, causing electrons to be emitted. It was discovered in 1887 by Hertz but was later explained by Einstein, who proposed that light is made up of discrete particle-like packets called photons. For a given metal, there is a minimum photon frequency, called the threshold frequency, required to cause electron emission. The kinetic energy of emitted electrons depends on the photon frequency and the metal's work function.