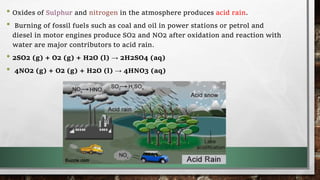
This document discusses various air pollutants and their effects. It describes gaseous pollutants such as oxides of sulfur, nitrogen, hydrocarbons and carbon, and particulate pollutants. It focuses on carbon monoxide and dioxide, explaining their sources and impacts. It then discusses global warming, its causes from increased greenhouse gases like carbon dioxide and methane, and impacts such as rising temperatures, sea levels and extreme weather. The document also covers acid rain formation from sulfur and nitrogen oxides, and its harmful effects on agriculture, plants, animals and aquatic ecosystems.