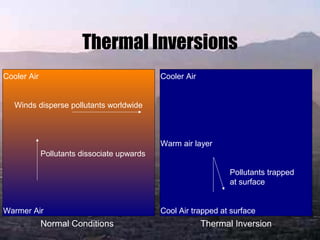
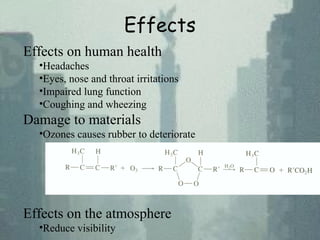
Smog is a mix of smoke and fog caused by air pollution. There are two main types: industrial smog from coal burning, containing sulfur dioxide and particulate matter; and photochemical smog from vehicle emissions containing ozone, nitrogen oxides, and other chemicals. Industrial smog causes respiratory issues and acid rain, while photochemical smog causes eye and respiratory irritation. Thermal inversions trap smog near the surface by warm air acting as a lid over polluted cool air. Smog has negative health, environmental, and economic impacts.