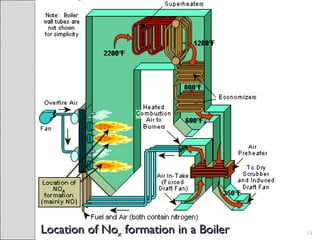
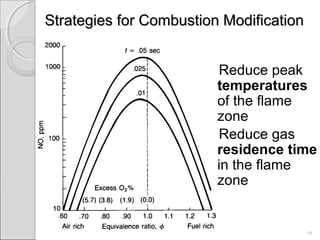
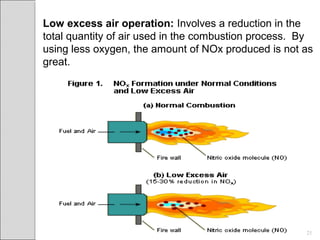
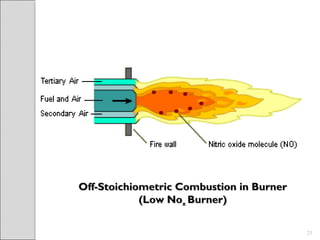
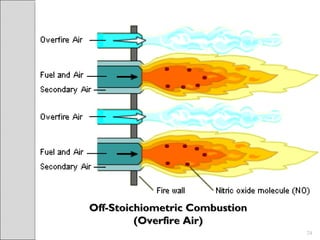
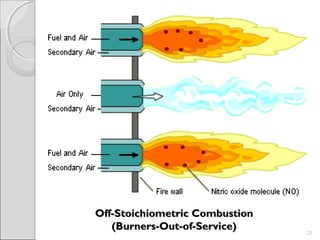
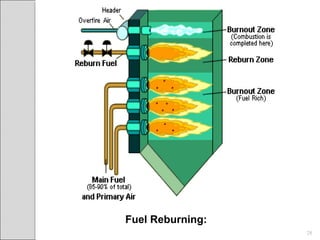
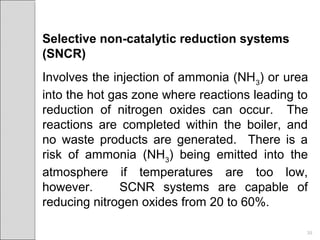
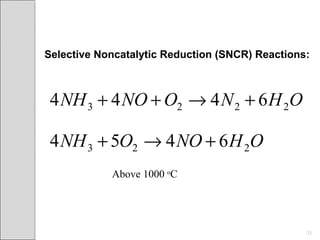
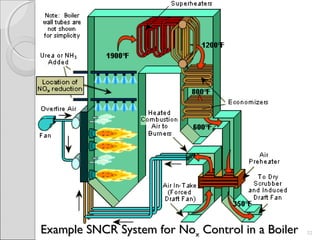
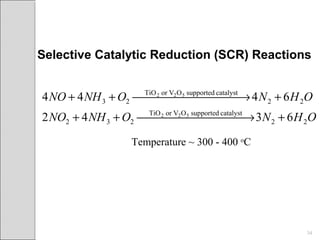
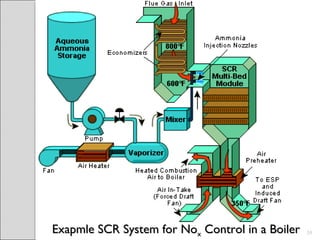
This document summarizes sources and control methods for nitrogen oxides (NOx). It discusses that NOx is primarily produced during high-temperature combustion processes from automobiles, boilers, and industrial operations. It has negative health and environmental effects. The document outlines various NOx control technologies including combustion modifications to reduce peak temperatures and residence times, as well as add-on controls like selective non-catalytic reduction, selective catalytic reduction, and dry sorption that chemically convert NOx to less harmful compounds. Wet scrubbers are deemed not suitable for NOx control since nitric oxide is insoluble in water.