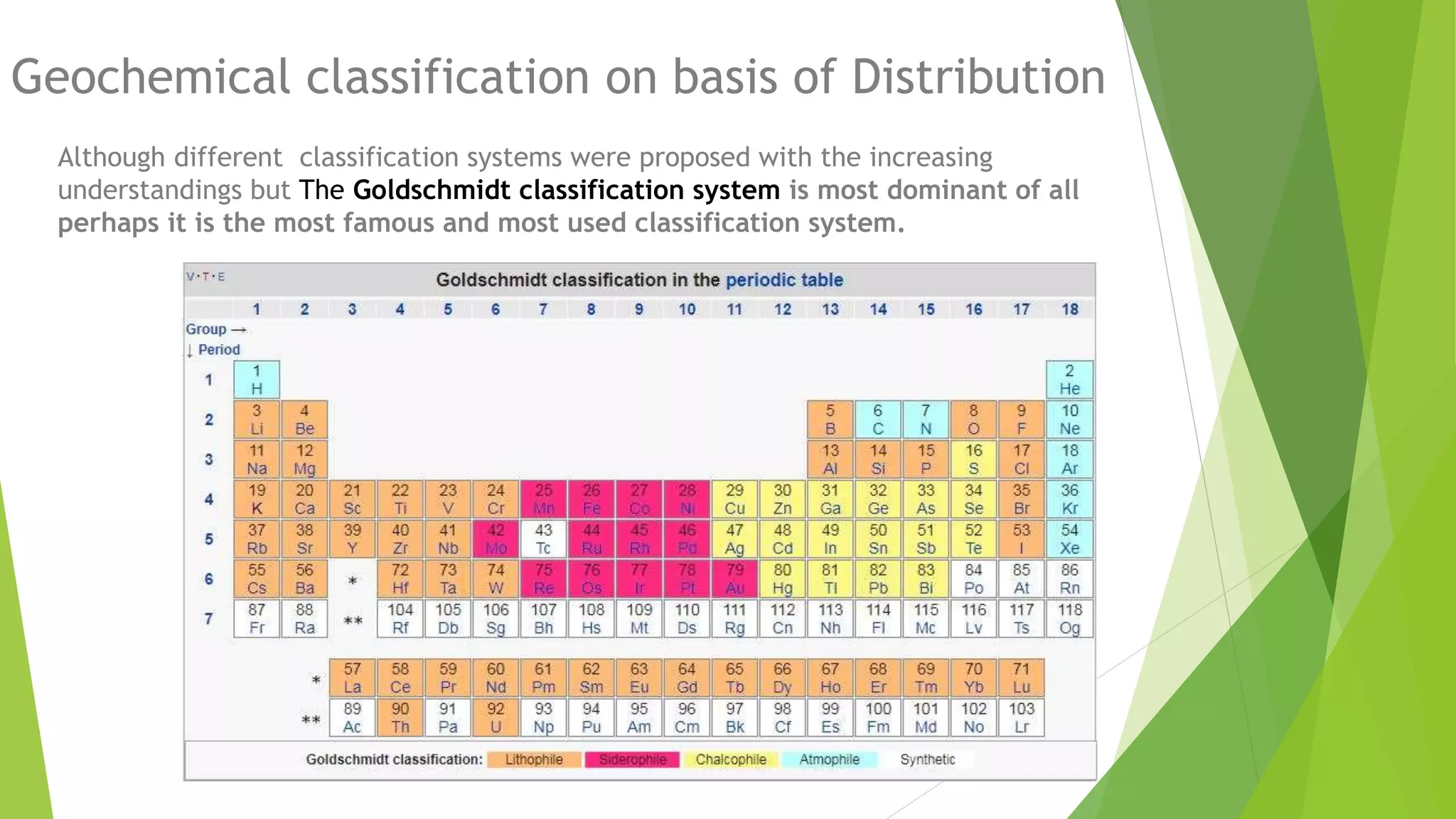
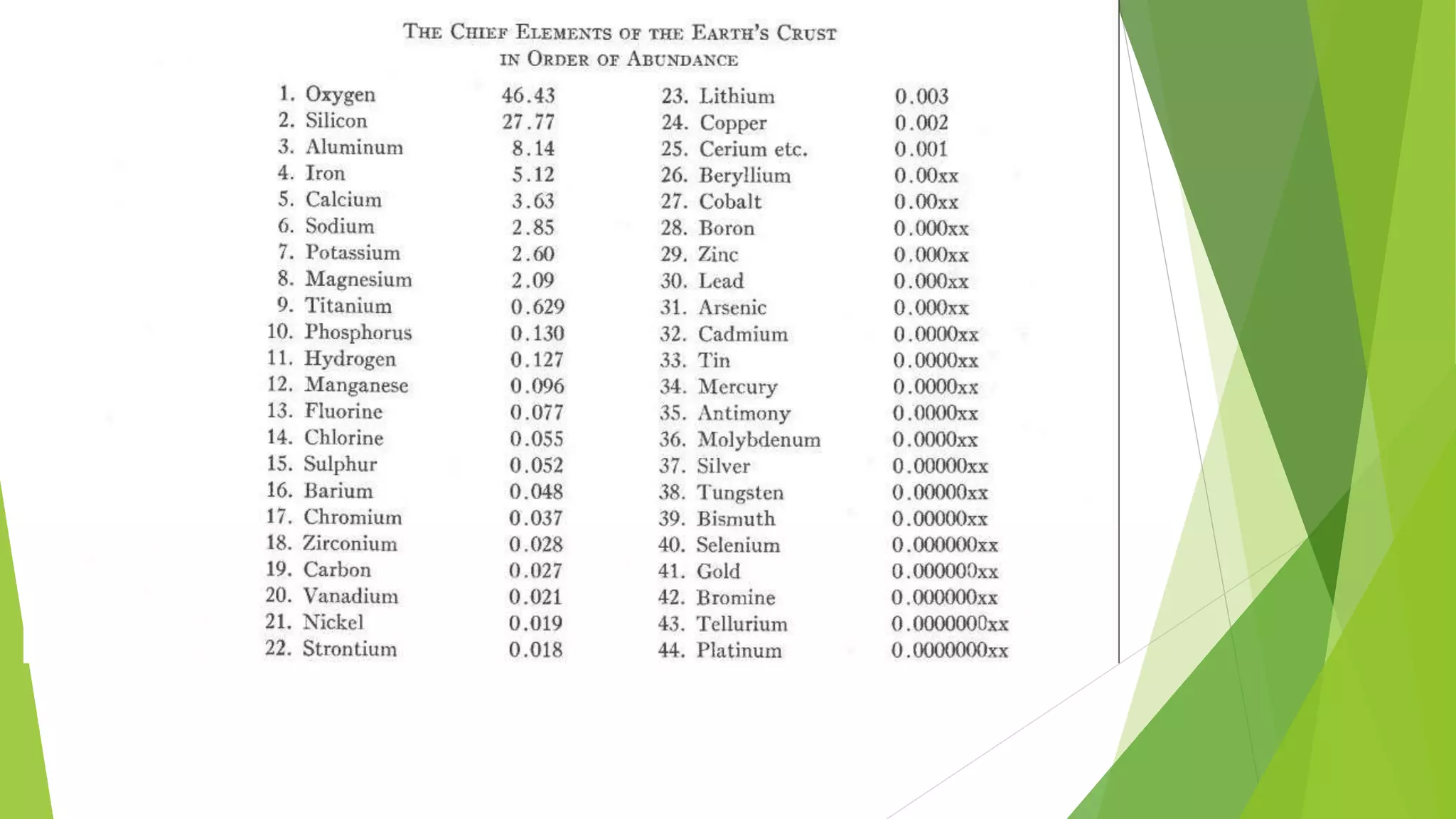
This document discusses the geochemical distribution of elements within the Earth's crust, emphasizing the importance of understanding the factors influencing the concentration of metals for mining and resource extraction. It highlights the contributions of researchers like Goldschmidt and Clarke, as well as defining the classification of elements into groups such as lithophile, siderophile, chalcophile, and atmophile based on their chemical properties and affinities. The document also explores various factors that affect these distributions, including rock type, ionic potential, temperature, and the processes of weathering and metasomatosis.