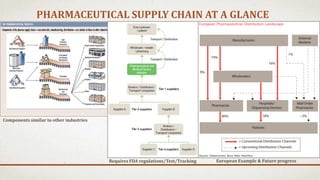
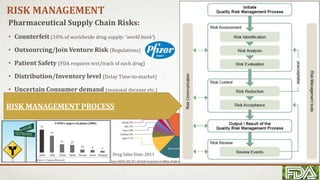
This document summarizes a study on supply chain practices in the pharmaceutical industry. It finds that the pharmaceutical supply chain is very fragmented, complex, and global. It operates in a competitive environment while also needing to ensure high regulations for patient safety. An effective supply chain can reduce costs and increase efficiency. However, it also faces risks like counterfeiting and needs strong risk management practices. The document outlines Pfizer's successful risk management efforts and FoxMeyer Drug Company's risk management failure which led to bankruptcy. Overall, the pharmaceutical supply chain is highly complex and regulated, with high costs of success or failure.