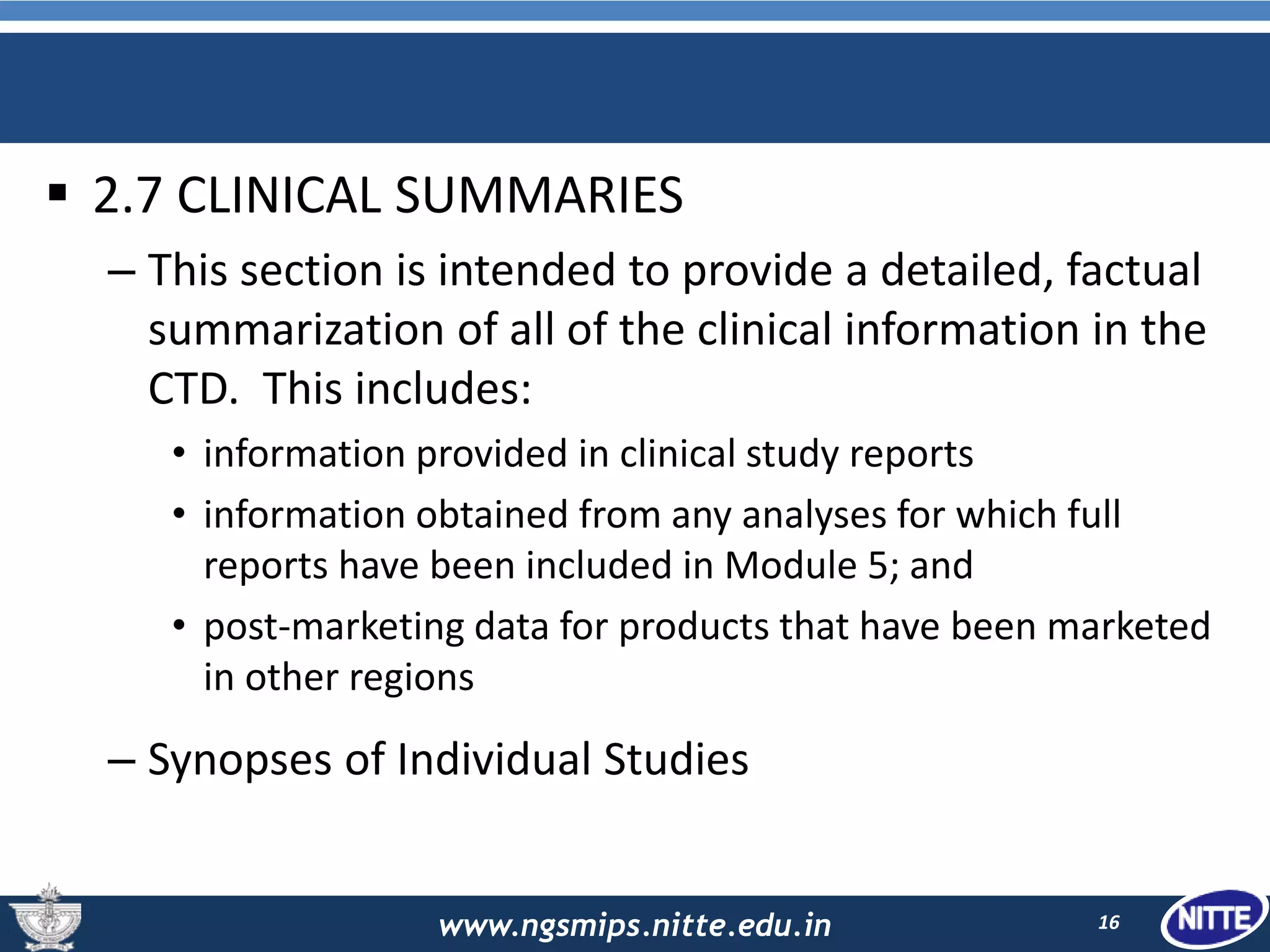
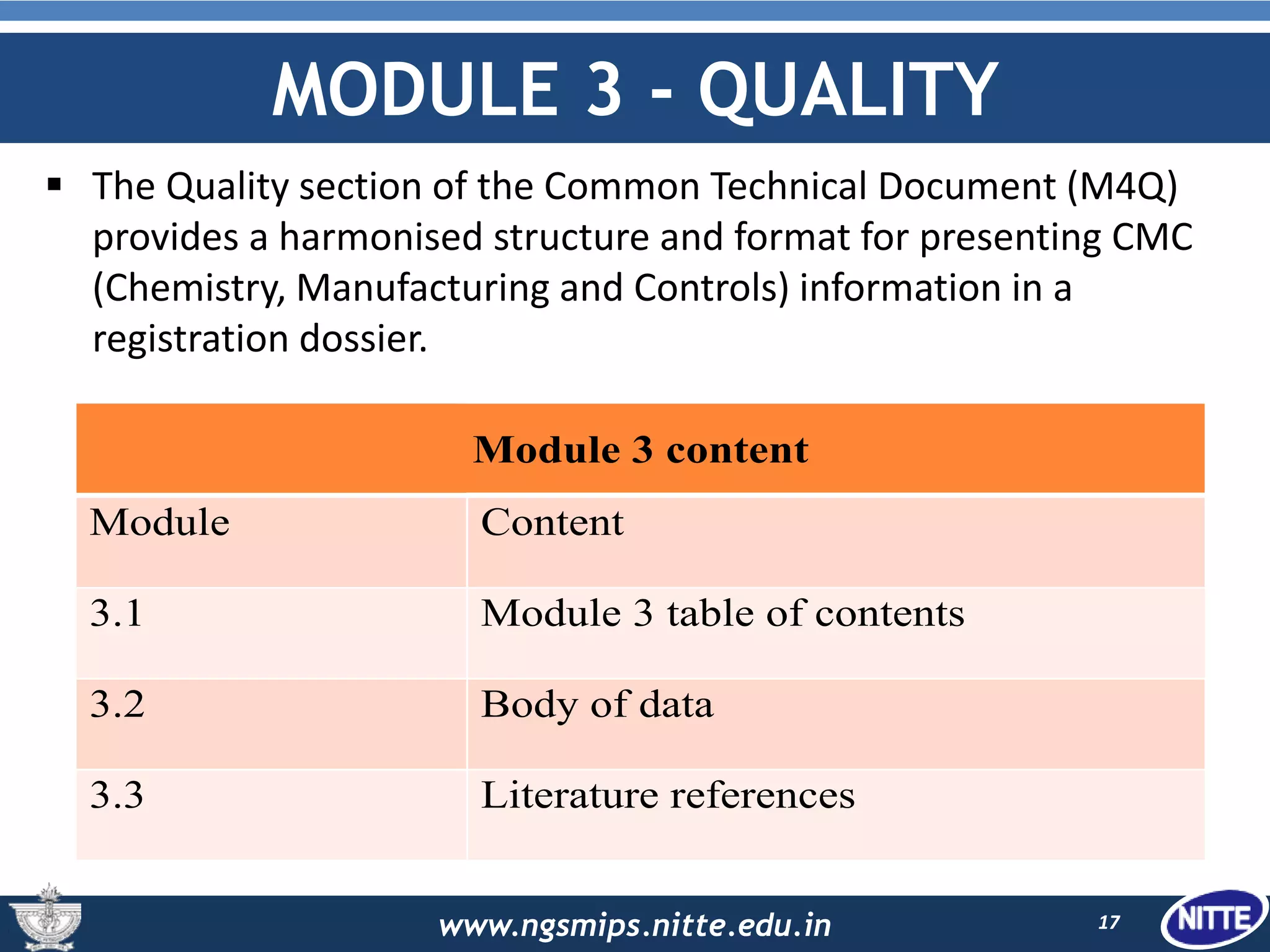
The document outlines the Common Technical Document (CTD), which is a standardized format created for the registration of pharmaceuticals in three key regions: Europe, the United States, and Japan. It details the organization of CTD into five modules covering general information, quality, non-clinical and clinical study reports, facilitating faster and more efficient submissions to regulatory authorities. The conclusion emphasizes the CTD's role in streamlining the application process, enabling simultaneous drug submissions across multiple regions.